Electric Vehicle (EV) battery recycling is crucial to a sustainable, electrified transportation system. A substantial portion of key minerals for electrifying could come from recycled batteries by 2050, dramatically reducing the need for new mining.
But how those batteries are recycled can make a big difference—we must use recycling processes with high mineral recovery rates and lower environmental impact. In this blog post, I’ll explain different ways to recycle batteries and why getting it right is essential.
The three types of recycling, summarized here, are discussed later in greater depth, including the pre-processing that must occur before recycling. This technology can be confusing and complex, so I have included a terms section for the italicized words at the end of this piece.
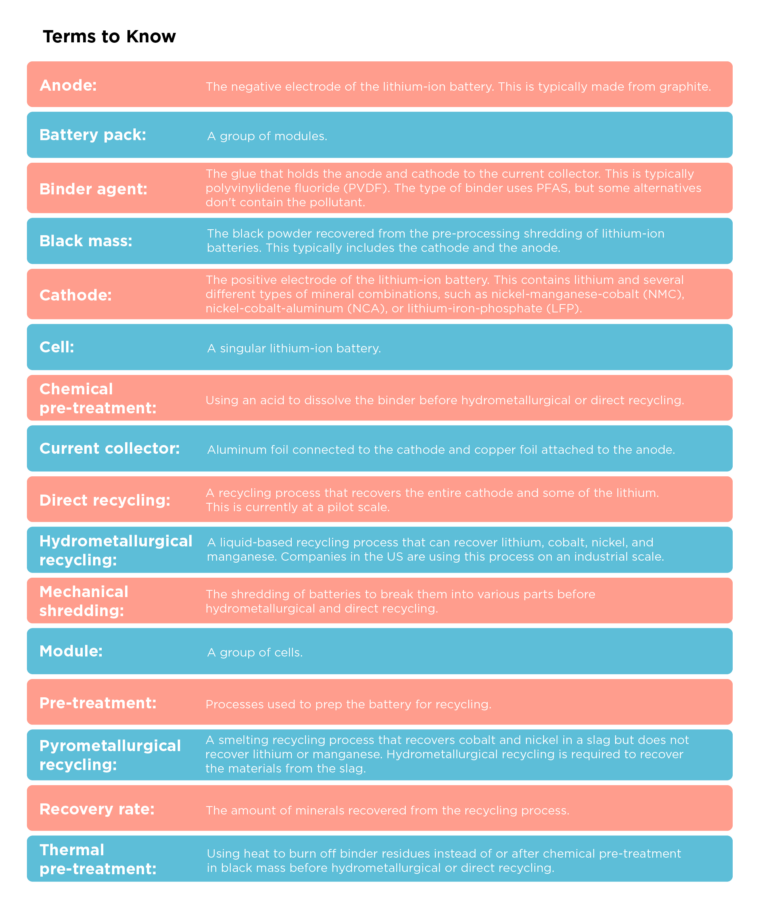
- Hydrometallurgical recycling is the best available technology because it has high mineral recovery rates and results in low environmental impacts. It uses liquid solutions to separate minerals.
- Direct recycling is still in development but has low environmental impacts and recovers the positive electrode intact, meaning this product skips a step in the battery manufacturing process. Direct recycling has lower lithium recovery rates than hydrometallurgical recycling but is ideal for manufacturing scrap and lithium-iron-phosphate (LFP) batteries.
- Pyrometallurgical recycling (smelting) is the least ideal technology because it does not recover lithium, aluminum, or manganese and results in the highest environmental impact. Additionally, the output must go through an extra step of hydrometallurgical refinement before it is ready for battery manufacturing.
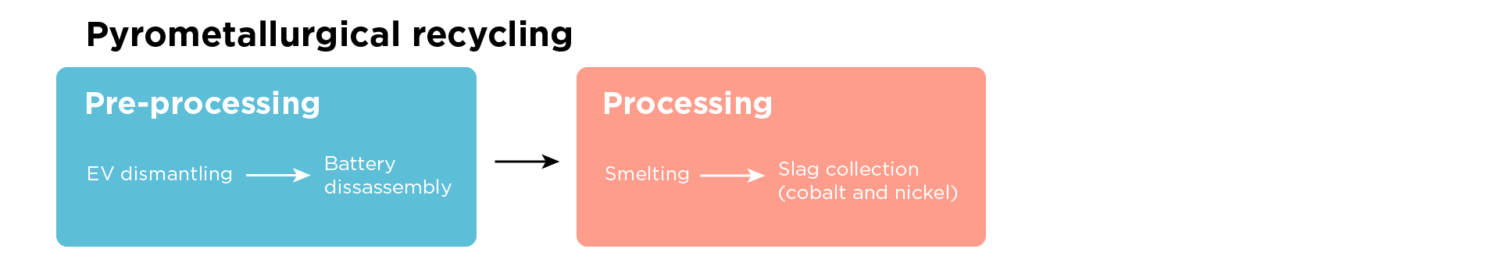
Pre-processing
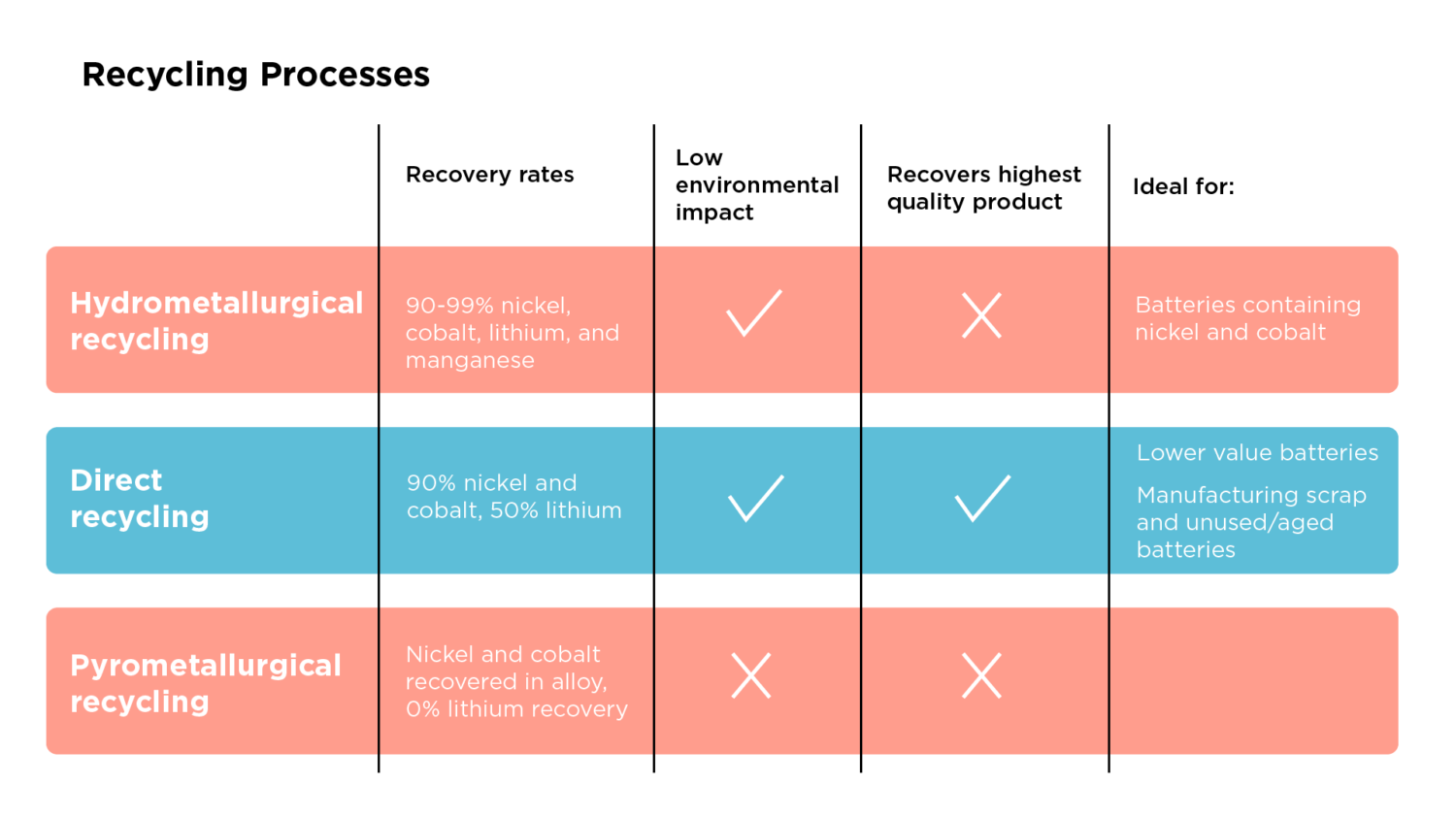
Before recycling, a car dismantler removes the battery from the EV and ships it to a battery recycler. The recycler takes the battery pack apart into smaller modules. These modules are typically rectangular and fit together in the pack, similar to a box of dominoes. Within each module are rectangular battery cells. Tesla uses a different design, where their cells are cylindrical instead of rectangular.
Pyrometallurgical recycling burns the whole battery; therefore, the material is ready for recycling after deconstruction to the module or cell level. Hydrometallurgical and direct recycling methods require separating the many cell layers from each other to get a higher-value product, requiring additional pre-processing.
In these additional steps, the module or cell is put through a mechanical shredder to break it apart into small pieces. The output is then separated through sifters, magnets, and shaker tables to isolate a powder that contains lithium, cobalt, and nickel (the powder is called black mass).
Hydrometallurgical recycling
- High recovery rates: 90-99% nickel, cobalt, lithium
- Low environmental impact
- Ideal for batteries containing nickel and cobalt
Hydrometallurgy uses liquid and chemical-based solutions to recover minerals from the powder that contains valuable metals (black mass) retrieved from the abovementioned pre-processing. The black mass powder contains the glue (binder) that sticks the positive electrode (cathode) and negative electrode (anode) to aluminum and copper foil (current collector).
Recyclers use several processes to separate electrodes from the foil, including the two broad categories of chemical pre-treatment and thermal pre-treatment. Chemical treatments use an acid to dissolve the glue, but additional processes, such as thermal treatment, must be followed to burn off residues. Thermal pre-treatment alone can also separate the positive electrodes from the foil through heating and burning off the glue and negative electrode. The burning process creates gases, so extra purification equipment, including scrubbers, is necessary.
This thermal treatment is technically considered a pyrometallurgical recycling process but significantly differs from the smelting explained in the pyrometallurgical section below. It uses lower temperatures that don’t change the state of the cobalt, lithium, and nickel we are recovering.
The recycler is then left with a powder containing the minerals. First, this is dissolved in a liquid (leaching), typically hydrogen peroxide and sulfuric acid. Next, the aim is to remove the valuable metals from the solution. One approach is solvent extraction. This technique uses two liquids with different solubilities (think oil and water) to separate the minerals naturally. This results in the minerals dividing with the liquids for accessible collection.
Wastewater created from this process must go through water treatment. In some operations (but not all!), they reuse this water in the facility. In addition, the process creates a large quantity of sodium sulfate, a low-value and safe byproduct used to make household items such as detergent.
The material output from hydrometallurgical recycling depends on the chemicals and processes used. Some companies’ final product is a positive electrode precursor, meaning they recover a mix of the minerals (positive electrode precursor), such as nickel-manganese-cobalt (NMC). Recovering these minerals together is ideal because the battery manufacturer who buys the recycler’s product does not have to make the positive electrode precursor. Other methods recover the individual materials (lithium, cobalt, and nickel sulfate, or carbonate salts) used to create a new electrode. The recovery rates vary by unique processes, but one company reports a recovery rate of 99% for cathode materials.
Direct recycling
- High recovery rates: 90% nickel and cobalt, 50% lithium
- Lowest environmental impact
- Recovers the highest quality product
- Ideal for:
- lower value batteries such as lithium-iron-phosphate
- manufacturing scrap and unused/aged batteries
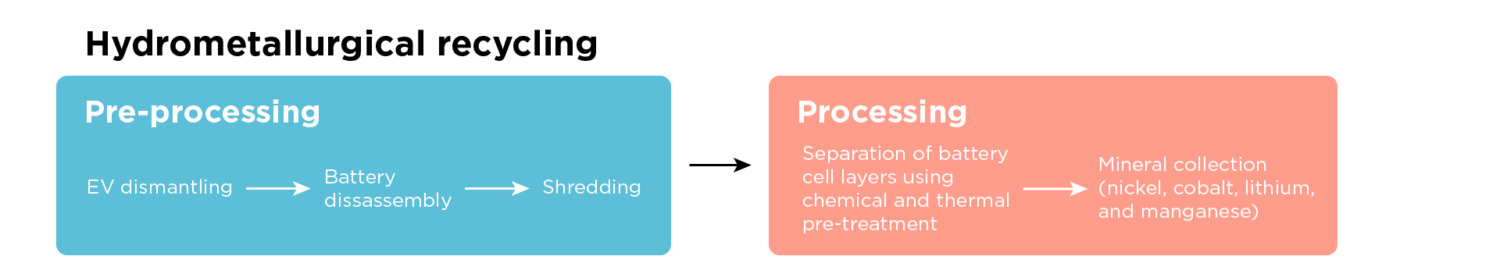
Direct recycling creates a rejuvenated positive electrode ready for manufacturing a new battery. This process is still in development but will likely be most favorable for batteries that don’t contain the high-value minerals of cobalt and nickel, such as lithium-iron-phosphate (LFP). The lower cost of inputs is part of the appeal of lithium-ion batteries that use LFP, making the recovery of individual minerals through hydrometallurgical recycling uneconomical. LFP is profitable if recycled using direct recycling because it recovers the positive electrode, making it a much more valuable product than only recovering the individual minerals.
For higher-value lithium-ion batteries, such as nickel-manganese-cobalt (NMC), direct recycling is most applicable for the waste material that comes directly from manufacturing because the crystalline structure of the electrode is damaged during the use of the battery.
Before recycling, the batteries are shredded. The black mass recovered is treated similarly to hydrometallurgical recycling, with slight changes to how the glue is removed and the negative electrode separated from the positive one. For example, instead of recyclers burning the negative electrode, typically graphite, they use the hydrophobic nature of graphite while heated to pull it to the surface of a liquid solution (the positive electrode is hydrophilic—repelled from the bubbles!). The graphite is in the foam at the top, and the other minerals stay below. This process is called froth flotation.
The recovered positive electrode does not have the necessary amount of lithium because of degradation and loss during its first use in a battery. Therefore, more lithium needs to be added (relithiation). In addition, as lithium-ion battery chemistries continue to evolve, and to ensure recycled materials are still usable, the recovered positive electrode can be modified or upcycled by adding additional minerals (e.g., adding more cobalt).
Pyrometallurgical recycling:
- Low recovery rates: nickel and cobalt recovered in the alloy, 0% lithium recovery
- Highest energy use and greenhouse gas emissions
Pyrometallurgical recycling is a broad category involving all technologies that use high temperatures to extract and purify metals. Several processes discussed for hydrometallurgical and direct recycling technically fit this category. Still, they use lower temperatures than the pyrometallurgical recycling in this section. This type of high-temperature pyrometallurgy is called smelting. Smelting does not recover all minerals and does require high energy usage to reach necessary temperatures.
Smelting heats battery materials above melting point to separate metals while in liquid form. Shredding the battery is unnecessary, as the battery module or cell goes directly into the furnace. The battery is first heated to 350-600* C to burn off the electrolyte and then heated around 1200-1450* C to melt the metals into an alloy. The recycler integrates off-gas treatment due to the toxic gases created in this process. The resulting metal alloy contains cobalt, nickel, copper, and iron. Smelting loses the lithium, aluminum, and manganese in the slag. The metal alloy must also undergo a subsequent hydrometallurgical process to recover the cobalt and nickel. The process loses a significant portion of the lithium as dust. While current recyclers aren’t pursuing it, an additional hydrometallurgical process can recover the lithium.
Battery recycling policy
A robust recycling policy would ensure that all EV batteries are safely recycled. Ideally, the United States would follow our global partners and enact extended producer responsibility (EPR). EPR holds automakers responsible for recycling all batteries. Recycling is a crucial step in a sustainable transportation system and supply chain, and automakers are poised to create a retired battery collection network and design batteries to be more easily recycled. But as this blog post points out, how they are recycled also matters!
Recyclers need to recover as much of the minerals as possible with the lowest potential impact, which means using a hydrometallurgical or direct recycling method instead of the pyrometallurgical alternative.
This recycling industry is quickly evolving with help from research institutes such as ReCell. Therefore, we suggest that policy sets baseline recovery rates instead of defining the technology pathway. For example, the European Union (EU) has taken this approach. The EU Battery Law requires the recycling processes to have a recovery rate in 2025 of 90% for cobalt, nickel, and copper and 50% for lithium, increasing to 95% and 80%, respectively, in 2030. Recycling policy is developing in California, and we hope to see the state apply a similar approach.