Available to watch now, The Electrochemical Society, in partnership with Hiden Analytical, Royal Society of Chemistry, ACS Materials Letters and JEOL USA, to gain nuclear magnetic resonance insights into ionic conduction in battery materials
Want to learn more on this subject?
 The development of next-generation solid-state ion conductors hinges on an understanding of microscopic diffusion mechanisms and the identification of roadblocks along macroscopic diffusion pathways (e.g. intragrain defects and grain boundaries). At the microscopic scale, ion conduction relies on transient short-range interactions between the diffusing and framework ions, and on the connectivity of the diffusion sites, hence, on the local structure and composition.
The development of next-generation solid-state ion conductors hinges on an understanding of microscopic diffusion mechanisms and the identification of roadblocks along macroscopic diffusion pathways (e.g. intragrain defects and grain boundaries). At the microscopic scale, ion conduction relies on transient short-range interactions between the diffusing and framework ions, and on the connectivity of the diffusion sites, hence, on the local structure and composition.
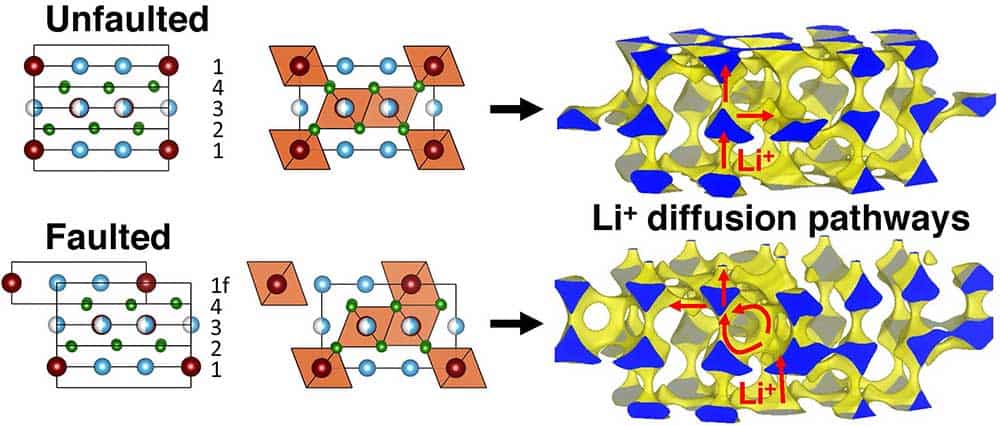
Two common assumptions in the design of solid electrolytes are that: 1) the absence of planar defects in the bulk of crystalline inorganic electrolytes; and 2) rapid polymer chain rearrangements (segmental motion) in polymer electrolytes; are required for fast ion diffusion. Yet, our recent work on Li- and Na-ion conducting rock salt halide electrolytes and polymeric ionic liquids demonstrates that these rules are relaxed for specific composition-structure combinations, such that fast ion diffusion on the order of mS/cm can be obtained in rock salt halides containing a significant fraction of planar defects, and in semi-crystalline polymeric ionic liquids.
Using a combination of electrochemical impedance spectroscopy (EIS), solid-state nuclear magnetic resonance (ssNMR), pulsed field gradient NMR (PFG-NMR), NMR relaxometry, and first principles calculations, we provide a multiscale understanding of ion diffusion processes and link these findings to local structure features, crystallinity and materials synthesis/processing conditions.
Want to learn more on this subject?
 Raphaële Clément is an assistant professor in the materials department at the University of California, Santa Barbara (UCSB), US. She received her PhD in chemistry in 2016 from the University of Cambridge, UK, working under the supervision of Prof. Clare Grey. Her doctoral work focused on the study of layered sodium transition metal oxide cathodes for Na-ion secondary batteries. She then joined Prof. Gerbrand Ceder’s group at the University of California, Berkeley (UC Berkeley), US, focusing on cation-disordered rock salt oxyfluorides for Li-ion battery applications. She joined the UCSB faculty in 2018. Her primary research focus is the development and implementation of magnetic resonance techniques (experimental and computational) for the study of battery materials and beyond, with a strong emphasis on operando tools. She is an Associate Editor for Battery Energy, a new open-access journal by Wiley.
Raphaële Clément is an assistant professor in the materials department at the University of California, Santa Barbara (UCSB), US. She received her PhD in chemistry in 2016 from the University of Cambridge, UK, working under the supervision of Prof. Clare Grey. Her doctoral work focused on the study of layered sodium transition metal oxide cathodes for Na-ion secondary batteries. She then joined Prof. Gerbrand Ceder’s group at the University of California, Berkeley (UC Berkeley), US, focusing on cation-disordered rock salt oxyfluorides for Li-ion battery applications. She joined the UCSB faculty in 2018. Her primary research focus is the development and implementation of magnetic resonance techniques (experimental and computational) for the study of battery materials and beyond, with a strong emphasis on operando tools. She is an Associate Editor for Battery Energy, a new open-access journal by Wiley.