One hundred years after “nuclear isomers” were first discovered, Philip Walker and Zsolt Podolyák pick five examples of these long-lived, excited nuclear states to show why they are so important in medical physics and beyond

It’s the late 1990s deep inside an underground data room at the Argonne National Laboratory near Chicago. The committee that awards beam time at the lab’s heavy-ion accelerator has approved an experiment in which we’ll fire a beam of uranium at a tungsten target. One of us (Philip Walker) and his PhD student Carl Wheldon have flown over from the University of Surrey in the UK to do the work.
Our uranium beam emerges from Argonne’s linear accelerator in pulses, exciting the tungsten nuclei to high energy, which then emit gamma rays as they decay. Most of the gamma rays are created whenever a pulse strikes the target, but for this experiment we’re more interested in what happens between pulses. In fact, we can watch the build-up of gamma rays on a screen in real time.
We know that some uranium–tungsten collisions will create nuclei like tantalum and rhenium, which will undergo beta decay – and that each daughter nucleus will then release its own gamma rays when it in turn decays. Because the shortest of these beta decays has a half-life of about 10 minutes, we reckon we’ll have to wait a few minutes before we see anything of interest on our screen. But then, whoosh! Within seconds, the gamma-ray signals are rising rapidly. What on earth is going on?
Initially, we wonder if the uranium beam is accidentally hitting the aluminium frame holding our tungsten target, rather than the tungsten itself. That would create lots of short-lived beta decays from nuclear reactions we hadn’t even thought about. But when we look closely at the energies of the gamma rays, it quickly dawns on us that the rays are coming from a form of tungsten-186 that had never been seen before. We’ve stumbled upon a new “nuclear isomer” – a long-lived excited nuclear state.
Since the first nuclear isomer was discovered in 1921 – exactly a century ago – nuclear physicists have discovered almost 2500 different examples of these excited nuclei with half-lives of at least 10 ns. But our discovery was even more exciting than usual, because most new isomers are found when unstable nuclei decay, whereas ours was created when a stable nucleus decayed. For isomer enthusiasts, it was something special.
Most isomers are fleeting objects, typically lasting less than a microsecond, which makes new examples hard to find. But with our pulsed beam at Argonne, we could investigate what happens between pulses, making it easier to pick out the gamma rays emitted by an isomer of interest from the mess of radiation created by other reactions. In fact, our study showed that it takes 3.5 MeV of energy to create our isomer by raising tungsten-186 from its ground to excited state. Later work showed it has a half-life of two seconds, although its other characteristics remain largely unknown to this day.
The birth of nuclear isomers
Nuclear isomers were discovered in 1921 by the German chemist Otto Hahn (1879–1968) while working at the Kaiser Wilhelm Institute for Chemistry in Berlin. It was the dawn of the nuclear age and scientists were still coming to terms with the discovery by the British chemist Frederick Soddy in 1913 of chemical “isotopes”. These are variants of chemical elements that have the same number of protons, but different numbers of neutrons – although neutrons were yet to be discovered.
Soddy had, however, also discussed the possibility of “a finer degree of isotopy”. Writing in a paper published in 1917, he hypothesized the existence of what he called “isotopes with identity of atomic weight, as well as of chemical character, which are different in their stability and mode of breaking up”. Soddy had, in effect, predicted what we now call nuclear isomers, though science historians are unsure if Hahn was directly inspired by Soddy’s work.

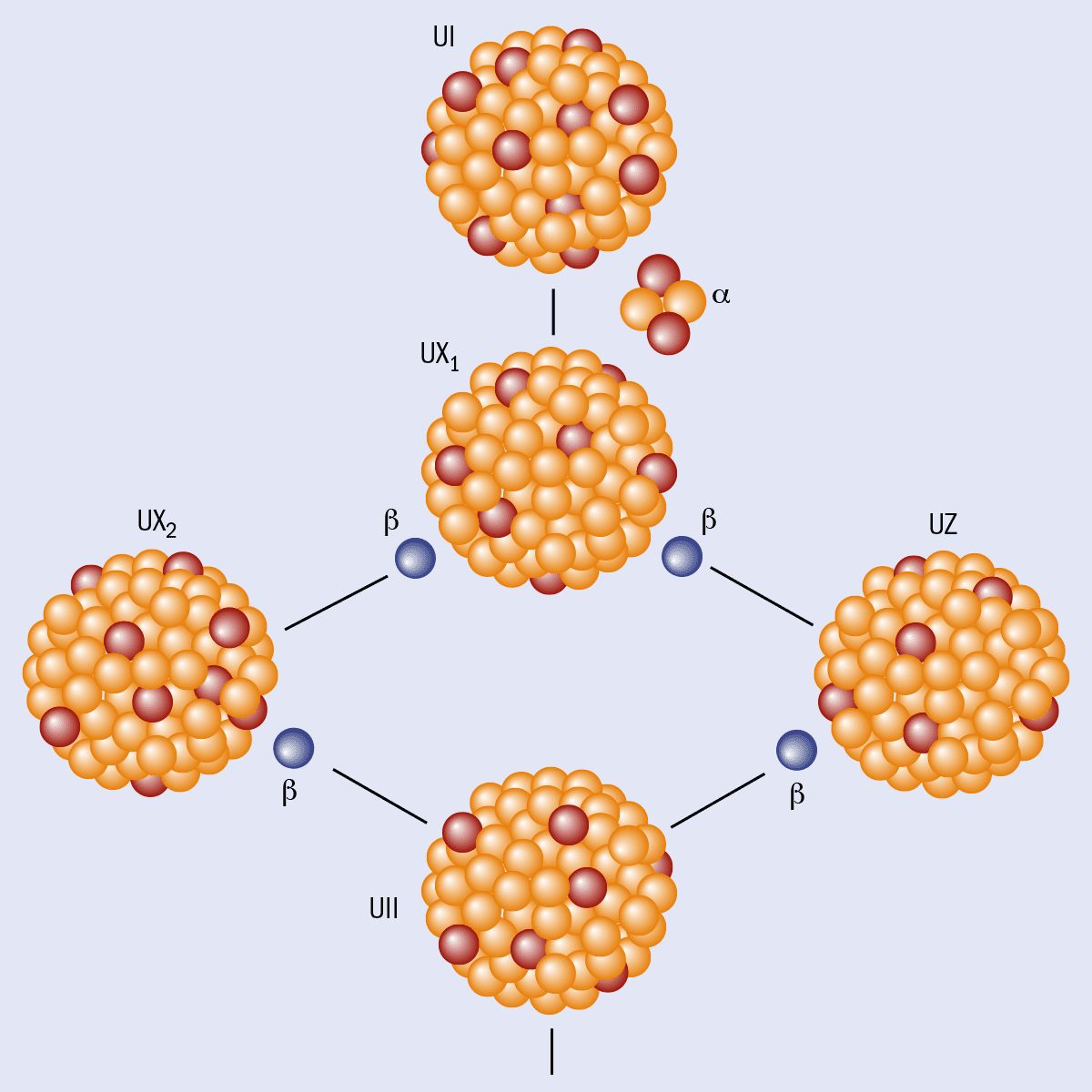
Instead, Hahn and his colleague Lise Meitner had been methodically mapping the complex process by which uranium-238 nuclei radioactively decay to form stable lead-206 nuclei. The decay chain involves a number of different nuclei, including a completely new chemical element with an atomic number of 91. Having discovered one particular isotope of this element, Hahn and Meitner dubbed it proto-actinium, now shortened to “protactinium”.
While looking back at his uranium-decay work in more detail, Hahn noticed something odd. What he called UI (uranium-238) can decay by emitting alpha particles to create UX1 (thorium-234), which then beta decays either into UZ (the ground state of protactinium-234) or into UX2 (an excited state of protactinium-234). Hahn, in other words, had discovered that protactinium-234 nuclei have two different states: a lower-energy ground state with a half-life of seven hours and an excited state with a half-life of one minute (figure 1).
Hahn’s work marked the discovery of nuclear isomerism and the birth of the new field of nuclear structure. However, progress in our understanding of isomers was slow and it was not until James Chadwick’s discovery of the neutron in 1932 that the concept began to catch on. Physicists now had theoretical ideas and experimental tools at their disposal to make sense of isomers, although the word itself did not appear in the scientific literature until a paper by the Ukrainian-born theoretical physicist George Gamow in 1934.
He suggested that the protactinium-234 isomer could be due to an antiproton in the nucleus – a concept that was greeted with considerable scepticism. It was 1936 before the German physicist Carl Friedrich von Weizsäcker came up with the accepted explanation for isomers. Von Weizsäcker realized that all nuclei have an angular momentum, or spin, and that different arrangements of the orbits of the protons and neutrons can create different spin states, just as “chemical isomers” have different spatial arrangements of atoms.
If the excited state has a very different spin from the ground state, it will take a long time to emit a gamma ray and decay to the ground state. In the case of protactinium-234, for example, the difference between the two states is four units of spin or 4ħ, where ħ is Planck’s constant divided by 2π. That makes the gamma decay so slow that the excited state is actually more likely to decay by emitting an electron (beta decay).
Uses and applications
A funny thing about isomers is that there is no agreed definition of the minimum half-life needed for an excited nuclear state to be an “isomer”. These states typically have a half-life of less than a picosecond (10–12 s) but some people say that isomers should live longer than a nanosecond (10–9 s) for them to count. But however you define them, isomers are only useful in a practical sense if they have a much longer half-life more like 1 s.
Thankfully, there are plenty that survive that long. Some are used in medical imaging, while others could potentially be used to store energy as “nuclear batteries” or to make super-accurate clocks (see box “Weird and wonderful: five applications of nuclear isomers“). Another interesting possibility is creating a new state of matter in which a gas of caesium atoms – whose nuclei are in an isomeric state – is cooled down to 100 nanokelvin to form a Bose–Einstein condensate (Phys. Lett. B 777 281). The atoms will be in their lowest-energy “condensed” state, but not the isomers, which are, by definition, excited states. Apart from being a bizarre and counterintuitive state of matter, you could potentially use it to create a “gamma-ray laser” by harvesting the coherent gamma rays given off when the isomers decay. Such a laser has never been made before.
An interesting possibility is creating a new state of matter in which a gas of caesium atoms is cooled down to form a Bose–Einstein condensate. The atoms will be in their lowest-energy state, but the isomers are, by definition, excited
Away from applications, spin isomers have been of fundamental importance in nuclear physics, especially to the “nuclear shell” model, which was developed in 1949 by Maria Goeppert Mayer and independently by Otto Haxel, Hans Jensen and Hans Suess. Just as electrons form atomic shells that can contain no more than a certain number of electrons, so neutrons and protons form nuclear shells, with similar limits to how many protons and neutrons each nuclear shell can hold.
The first can have up to two, the second goes up to eight, with subsequent shells having a maximum of 20, 28, 50 and 82 nucleons. Known as “magic numbers”, they have the same values for both proton and neutron shells, although neutrons have an additional magic number of 126. But the parallels between the electron and nuclear shell models aren’t perfect because while the spin-orbit force between electrons is weak and repulsive, the spin-orbit nuclear force is strong and attractive. This affects the spin structure, in particular making it more likely for isomers to form when the shells are full or almost so.
Nuclear physicists have also recently discovered that the proton magic numbers (2, 8, 20 and so on) can vary depending on how many neutrons are present and vice versa for neutron magic numbers. Given that the original magic numbers were found in nuclei that were stable (or nearly so), the fact that they might not be so magic after all has forced us to rethink our ideas about the structure of unstable nuclei, with isomers playing a key role in that quest.

Hunt for the superheavies
Stranger and stranger
A century after nuclear isomers were discovered, there is plenty still to investigate. We’ve mentioned that nuclear isomers can exist if there are large changes in nuclear spin. But remember that spin, being a vector quantity, has a direction as well as a magnitude. In fact, there’s another type of isomer that depends on the change in spin direction. First understood in 1955, we now know of more than 100 such “K-isomers”, which often exist in heavy, deformed rugby-ball-shaped nuclei. The spin typically points along the long axis of the nucleus, but when the isomer decays, the spin in the populated state usually is at 90° to the axis.
There is also another type of isomer in which the excited nucleus changes shape significantly when it decays to the ground state. First discovered in the early 1960s, we now know of about 50 such “shape isomers”, which can go, for example, from being round to prolate. In very heavy nuclei, there is even a subset of shape isomers, known as “fission isomers”, in which the excited nucleus spontaneously splits into two lighter nuclei when it decays. Since fission limits how heavy a nucleus can be, fission isomers have been vital in helping us to understand the stability of heavy nuclei.
The heaviest element made in the lab so far is oganesson-118, which has an atomic number of 118 and is named after the Russian-Armenian physicist Yuri Oganessian, who discovered it in 2006. Wouldn’t it be great if nuclear isomers could help us to find even heavier elements still? And given that isomers can even help us understand how stars explode and create life-giving chemical elements here on Earth, we can truly say that isomers give us a window onto our origins.
Weird and wonderful: five applications of nuclear isomers
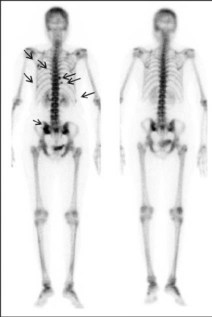
Medical imaging
Many different radioisotopes are used in medicine for both diagnostics and therapy. The most widely employed, with about 20 million applications a year, is an isomer of technetium-99. It’s ideal for imaging, emitting a single 141 keV gamma ray with no accompanying beta particles. Its six-hour half-life, meanwhile, is long enough for a scan of a particular organ to be carried out, but short enough to decay away fast, thus reducing the overall dose to the patient. Used as a tracer in bone, brain and heart scans, the technetium-99 isomer is introduced into the body as part of a molecule specifically chosen to have an affinity for the organ of interest. The rapid decay, however, means that the isomer cannot be stored, forcing hospitals to order its parent nucleus – molybdenum-99 – instead. Created from the fission of uranium-235 in nuclear reactors, molybdenum-99 has a much longer half-life of 66 hours and can easily be transported around the globe. It decays into technetium-99, which hospital staff chemically extract from the molybdenum/technetium mixture.
Nuclear clocks

The isomer with the lowest energy we’re aware of is thorium-229, which has an energy of just 8.1 eV, corresponding to light with a wavelength of 150 nm. Tremendous progress has recently been made in understanding its properties, but even measuring its excitation energy has been a challenge, requiring the development of new types of radiation detector. The half-life of neutral thorium-229 atoms is 7 μs but calculations suggest its ions could survive for many orders of magnitude longer. That possibility has led physicists to propose many potential applications, including building a clock that is more accurate than any other (Nature 573 202) and finding out if the fundamental constants of nature vary with time.
Super-heavy elements

Elements with an atomic number, Z, of 104 or above are called super-heavy elements. Short-lived, they do not exist naturally on Earth, but can be synthesized in laboratories by fusing together lighter nuclei. Super-heavy elements are important for nuclear physics, atomic physics and chemistry, with the heaviest element created to date being oganesson (Z = 118). But the heaviest element with a known isomeric state is darmstadtium (Z = 110), which was first created in 1994 at the GSI lab in Darmstadt. The darmstadtium-270 isomer has a half-life of 4 ms, meaning it is much more stable than the ground state (half-life of just 0.2 ms). If this principle holds more widely, then isomers could play a crucial role in the discovery of new and even heavier nuclei (Phys. Rev. Lett. 92 252501).
Nuclear batteries

Nuclear isomers could be used to make a new kind of super-charged battery as they have energies of several mega electron volts (MeV) per atom, which could be released by shining light on them. One possibility would be to use tantalum-180m – one of two naturally occurring isotopes of tantalum. It is the longest-lasting isomer we know of, having a half-life longer than the age of the universe. This isomer, which has an excitation energy of 75 keV, can release its energy by shining 1 MeV photons onto it. But as that energy is rather high, one promising alternative is the molybdenum-93 isomer, whose stored energy of 2425 keV can be released by exciting it with photons of just 5 keV (Nature 554 216). Unfortunately, it has a half-life of only seven hours, which is why researchers are also looking at the americium-242 isomer. It stores an energy of 49 keV, has a half-life of 141 years and its energy could be released by exciting it with photons of just 4 keV.
Exploding stars
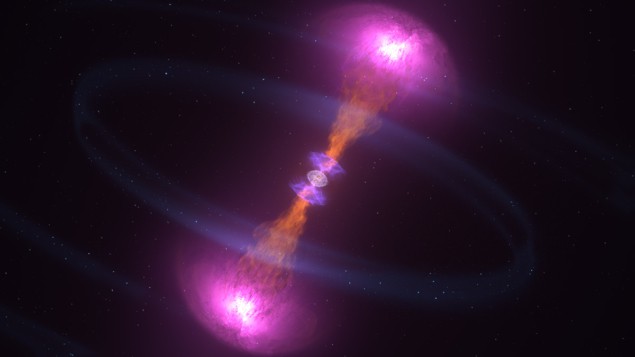
Roughly half of every element in the solar system that lies above iron in the periodic table was made in colliding or exploding stars. Precisely how is a matter of debate, but neutron-star mergers and supernova explosions are the two most likely possibilities. So extreme is the environment in such locations that nuclei with enormous neutron-to-proton ratios are created, most of which have never been produced on Earth. Nevertheless, a few of them can be studied in accelerator laboratories. One example is the palladium-128m isomer, which has 46 protons and a magic number (82) of neutrons (Phys. Rev. Lett. 111 152501). With a half-life of 6 μs, it lasts long enough for the isomer to be whisked away from the accelerator where it’s made and studied in low-background conditions. Such studies have shown that 82 remains a robust “magic number” (see main text), with palladium-128m playing a central role in the synthesis of new elements in exploding stars.