Scientists in Australia have used cryo-electron microscopy to study the structure of protein anchor chains that keep cells in place in the human body. These microscopic structures, 1/10,000th the width of a human hair, help cells pull and push on their environment, and are important for cell migration. Certain types of cancer cells – having fewer anchor chains – can disengage from their environment and move more freely throughout the body, a process known as metastasis. The number and distribution of these anchors can thus be used to detect whether cancer cells are likely to spread.
The researchers, led by Peter Gunning and Edna Hardeman at the University of New South Wales Sydney, describe their findings in Nature Materials.
Most cells in the human body are attached to the extracellular matrix – the protein scaffold that gives the body its structure – via small links known as “focal adhesions”. Microscopic motors move along the fibres attached to these adhesions and allow cells to sense the stiffness of the surrounding matrix. Under suitable stiffness conditions, normal cells proliferate; or else they undergo programmed death.
Cancer cells, however, are known to evade programmed death, and instead proliferate and migrate throughout the body.
“We’ve identified the protein that’s essential for these attachments to function,” explains Maria Lastra Cagigas, first author of the study. “If these attachments fail, the cell could be more prone to moving and invading tissues, like cancer.”
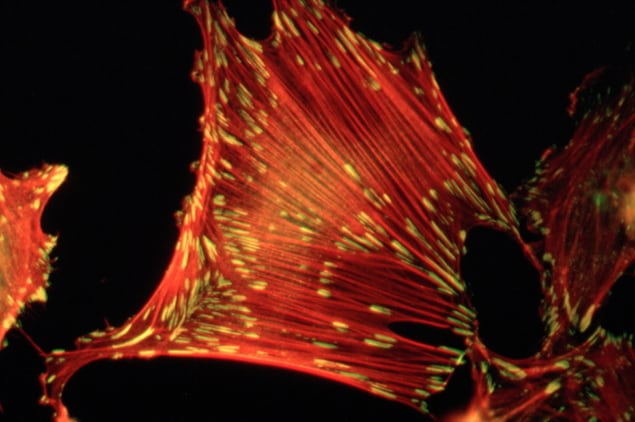
Using specialized cryo-electron microscopy, Lastra Cagigas and co-authors compared the presence and distribution of several variants of the protein tropomyosin – the key component of these anchor chains – in healthy mouse cells and human cancer cells.

Tagging tropomyosin with a fluorescent molecule, they took snapshots of the different cell types from different view angles using an electron microscope and combined these snapshots into a 3D picture of the cell.
The researchers found long protein chains composed of tropomyosin and actin running from the centre of the cells to their edges, where focal adhesions were present. Cancer cells had fewer focal adhesions, and the protein chains were farther apart than in normal cells. When the researchers put back the tropomyosin into cancer cells, they found that the cells recovered the normal number of adhesions.

The rise and rise of cryogenic electron microscopy
Senior author Gunning explains that this is a fundamental biophysical principle used by cells to solve a major structural problem in biology. “This fits into our growing realization that all animal cells use different variant forms of tropomyosin to build chains with different functional properties. We are now using this technology to visualize how the tropomyosin building blocks are assembled into these chains,” he tells Physics World.
In the long term, the researchers hope to use their understanding of focal adhesions to also design drugs that target these tropomyosin chains in certain types of cancer where cancer-support cells build a barrier around the tumour to protect it from anti-cancer therapy. Gunning and Hardeman have established a company focused on developing drugs that target different tropomyosins.



