Sarah Vitak: This is Scientific American’s 60 Second Science. I’m Sarah Vitak.
If you are kitchen-savvy, you probably know “the water test” method for getting stainless steel pans to the right temperature.
[CLIP: Sizzling sounds]
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
All you have to do is splash a few drops of water in a pan as it is being heated. When the pan reaches the right temperature, instead of evaporating, the drops form into beads and skate all over the pan.
You have to do this test in an empty pan. And that is because, as the not-kitchen-savvy among us may have learned the hard way, mixing oil and water in a hot pan is a recipe for flying burning hot liquids. The phenomenon that happens when beads of water skate around in your empty pan has been understood for a long time. Here is Dr. Kripa Varanasi from the mechanical engineering department at M.I.T. explaining it:
Kripa Varanasi: They’re levitating on their vapor layer with the vapor cushion that’s forming because the liquid is evaporating. And so they can move around with very little friction: that’s called the Leidenfrost state. And then, if you’re at a lower temperature, you could start boiling the drop, but the drop is sticking to the surface.
Vitak: But recently Dr.Varanasi and his graduate student Victor Leon set out to look at hot oil and water interactions a bit more closely.
Varanasi: What we found here that’s very interesting is: When you have a thin oil layer, you have the drop on it, you still can get both those states. But in between, there is another state, and it can cause it to race at very high speeds on the surface.
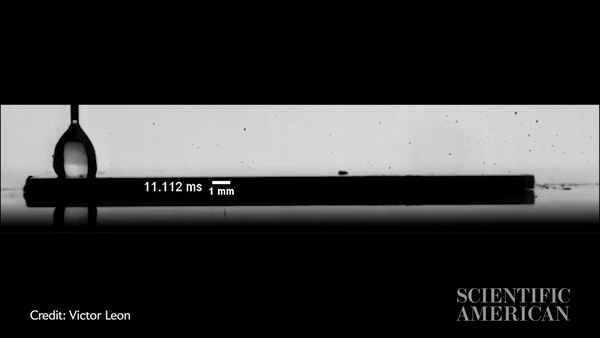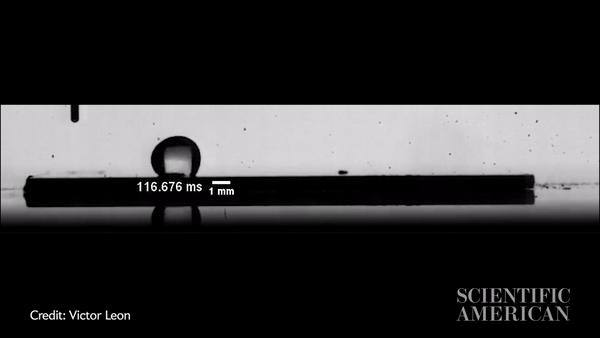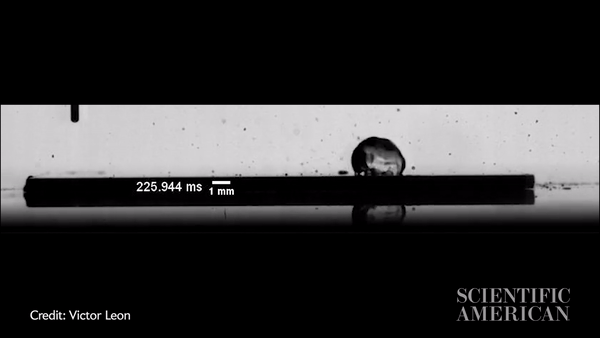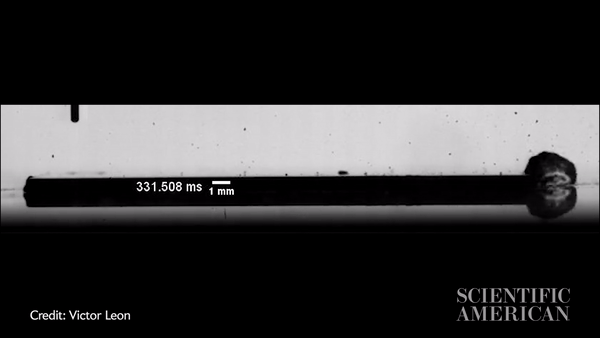
Slow-motion video showing a water droplet moving across an oil-coated silicon wafer in just more than 300 milliseconds. Credit: Victor Leon
Vitak: The experiments were so simple, you could run them in your kitchen. They just used a hot plate with either a metal plate or a silicon wafer on top, then the oil (they used silicon oil), then a syringe to precisely place a water drop. The one thing they did a little more precisely than you would in the kitchen is that they applied the oil by spin coating, a process that creates a very thin and very uniform surface. Their oil layer was about 10 microns, or about a hair’s width thick. They used high-speed imaging equipment to capture the result.
And what they saw in these videos was that when the droplets were placed on the hot oil, they would propel themselves in random directions at very high speeds. [Victor Julio Leon and Kripa K. Varanasi, Self-propulsion of boiling droplets on thin heated oil films]
Several things about this were very surprising. First, that the droplet propels itself. It was clear pretty quickly how this was happening.
Varanasi: An easy way to think about this is it's called momentum transfer. So if you take a balloon and blow air in it, and you release it, the balloon just flies everywhere, right?
Vitak: Similar to the balloon, what was happening on the hotplate was that the oil was forming a thin layer around the drop. The oil cloak acts as the balloon. The water inside boils and turns into vapor. Eventually, this pocket of steam bursts through the oil, and much like letting go of the balloon this propels the drop.
The second thing that confused them was that the drops went much faster than those levitating leidenfrost water drops. Ten to 100 times faster, in fact. That was odd because the drop was still in contact with the oily surface. You might expect to slow it down compared to a levitating drop.

Video shot at 100,000 frames a second shows steam inside an oil-coated water droplet ejecting smaller water droplets at high speeds. Credit: Victor Leon
Victor Leon: And so we were pretty stumped because the velocity of the droplet actually seemed that it moving on an infinite pool of liquid.
Vitak: That is Leon who ran the experiments. He means that when he did the back calculations based on the speed that he saw the water drop was moving, the only way to make the calculation work out would be for the drop to be on an infinitely deep pool of oil. But in reality the oil was only 10 microns thick, which should cause the drop to stick--a lot.From a physics perspective the amount of friction the oil surface should have is based on its depth. Think of it like pouring honey out of a bottle. The thin film is like pouring through the small opening in the cap -- the honey moves very slowly. If you remove the cap entirely - the honey has less friction and pours more quickly. If you do the calculation for oil 10 microns thick the drop should move way slower.
Leon: I mean, that was one of the moments where I was like, No way like, This is crazy.
Vitak: But what was even more surprising was the explanation. And that came when they looked at the super slow motion videos.
Leon: And we actually saw the surface of the droplet moving at this frequency that suggested that it's vibrating due to boiling events like bubbling.
Vitak: So as the water inside the drop boils bubbles form. The bubbles trapped inside the oil film cause the drop to go from perfectly circular to changing rapidly between asymmetric shapes. It looks like a sort of chaotic vibrating. And it means that the bottom of the drop is changing between different wavy shapes and no longer fully in contact with the oil the way it would be if it stayed as a perfectly circular drop. So the friction is much lower. And the drop can go really fast.
Varanasi: These are things that you see in very complex physical systems. So we are like, quite both first, quite stumped in the beginning to then later on, delighted by figuring this out.
Vitak: But they don’t have it all figured out yet. There is one question in particular they want to look at next:
Varanasi: But why does the vapor eject from one side, right? Or how does it choose that? That's still a mystery for us. And we are figuring that out. Because once we know that we can then direct the motion of the drops to the way we want them.
Vitak: If they can harness this power these forces could be used for many different applications. From handling tiny amounts of liquid for biotech applications, to cleaning films that build up in water treatment, to moving liquids in microgravity on other planets, to even carrying molecules to make tiny robotic delivery systems. And it all started as a flash of insight in the frying pan.
Thanks for listening. For Scientific American’s 60 Second Science, I’m Sarah Vitak.
[The above text is a transcript of this podcast.]