CLIMATEWIRE | A team of scientists from Massachusetts has developed a process to convert one of the world's most threatening planet-warming emissions — carbon dioxide — into a powdery, harmless fuel that could be converted into clean electricity.
The breakthrough follows an almost centurylong effort to turn CO2 into a cheap, clean fuel. Researchers at the Massachusetts Institute of Technology exposed CO2 to catalysts and then electrolysis that turns the gas into a powder called sodium formate, which can be safely stored for decades.
“I think we have a big break here,” said Ju Li, an MIT professor leading the research team. “I could leave 10 tons of this stuff to my granddaughter for 50 years."
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Researchers have previously turned CO2 into fuels that required too much energy to make, or were difficult to store long term.
The MIT process gets closer to an ambitious dream: turning captured CO2 into a feedstock for clean fuel that replaces conventional batteries and stores electricity for months or years. That could fill gaps in the nation's power grids as they transition from fossil fuels to intermittent solar and wind energy.
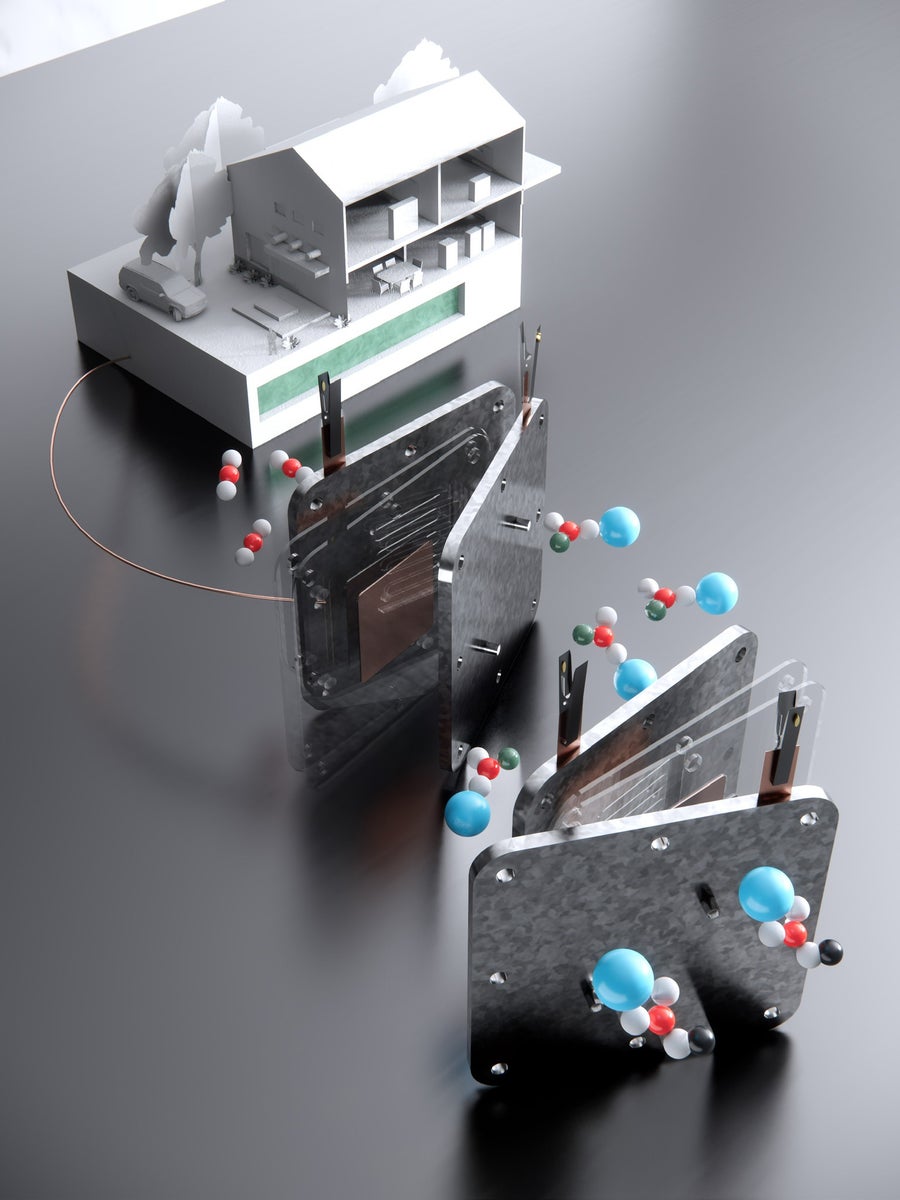
A schematic shows the formate process. The top left shows a household powered by the direct formate fuel cell, with formate fuel stored in the underground tank. In the middle, the fuel cell that harnesses formate to supply electricity is shown. On the lower right is the electrolyzer that converts bicarbonate into formate. Credit: Image: Shuhan Miao, Harvard Graduate School of Design
But the effort has been an uphill battle. A 2018 study called CO2 a “notoriously inert molecule;" two years later, another paper declared the invisible gas as “far more pernicious” to work with than researchers had thought.
The MIT team traces its breakthrough to November 2022. That's when Li, who started his career as an undergraduate at China’s University of Science and Technology, went to a conference of the school’s alumni in Boston.
The 48-year-old Li met Dawei Xi, a young doctoral student in engineering at nearby Harvard University. Xi, now 27, was skeptical of the conversion efficiency of captured CO2, predicting that the team's efforts would make a fuel that was too acidic.
“We were arguing on basic electrochemistry,” Li recalled. “He provided much valuable guidance on how to do this.”
Xi eventually joined the research team, and Li introduced him to Zhen Zhang, one of his graduate students. Xi explained that his hunch was that the MIT process would became ”acidity imbalanced,” making the product useless after a short period of time.
Within a month, the pair had identified the problem and worked out what later proved in the MIT laboratory to be a highly efficient way to convert captured CO2.
The resulting powder closely resembles a commercial product that has been safely used for years to melt ice on highways and airports. It has been stored for 2,000 hours in tanks without a hint of corrosion, Li said.
Li's team has also designed a refrigerator-sized fuel cell that uses a liquefied version of the stored power. That could produce electricity for homes, he said, and “nothing goes into the atmosphere.”
“Think of it as artificial wood,” Li said.
Li said he is beginning discussions with commercial companies interested in the MIT process that emerged. Li's team is also exploring ways heavy industries might use it to meet company CO2 emission reduction goals.
So what happens next?
“There is this valley of death,” Li noted, using a term scientists often use to describe the difficult process of scaling up a laboratory solution into a commercial product.
“We will need space and money," he said, "and that’s not easy to do in a university.”
Last month, Li's team published a study in the journal Cell Reports Physical Science outlining their efficient process for converting CO2 into fuel.
“Several improvements account for the greatly improved efficiency of this process,” said Zhang, the study's lead author. That, he said, improves the prospect of CO2 utilization for long-term energy storage.
A fuel derived from CO2, Li said, could be more promising than hydrogen and methanol for power generation. Methanol is a “toxic substance” and its leakage could cause a “health hazard," Li said, while hydrogen gas can leak from pipes and tanks, “precluding” the possibility of long-term storage.
Reprinted from E&E News with permission from POLITICO, LLC. Copyright 2023. E&E News provides essential news for energy and environment professionals.
