The rate at which water molecules cluster together to form ice crystals can vary depending on which “nucleation seeds” are introduced, researchers in Japan have discovered. Through high-resolution imaging experiments, a team from Panasonic Corporation, Osaka University and Waseda University found that crystallization in water-based materials is accelerated by the addition of silver nanoparticles – suppressing the often-inconvenient effect of supercooling. The discoveries could pave the way for improvements in heat-storing materials.
When water is cooled below its freezing point, molecules will quickly start to cluster around impurities in the liquid. Through the process of nucleation, these clusters become larger and more frequent, before eventually amalgamating to form solid ice crystals. Nucleation can be inhibited when water contains few impurities, and is left undisturbed during cooling – allowing it to maintain its liquid state even below its freezing point.
This supercooling effect can impair the performance of latent heat storage materials. In these systems, heat can be extracted or stored in frozen water-based solids, without changing their temperature – but this cannot be done efficiently if the materials are still liquids below their regular freezing points. This inconvenience is now driving research into how supercooling can be supressed, allowing nucleation clusters to form more readily. Currently, however, the mechanisms by which these clusters form around impurity seeds are still poorly understood.
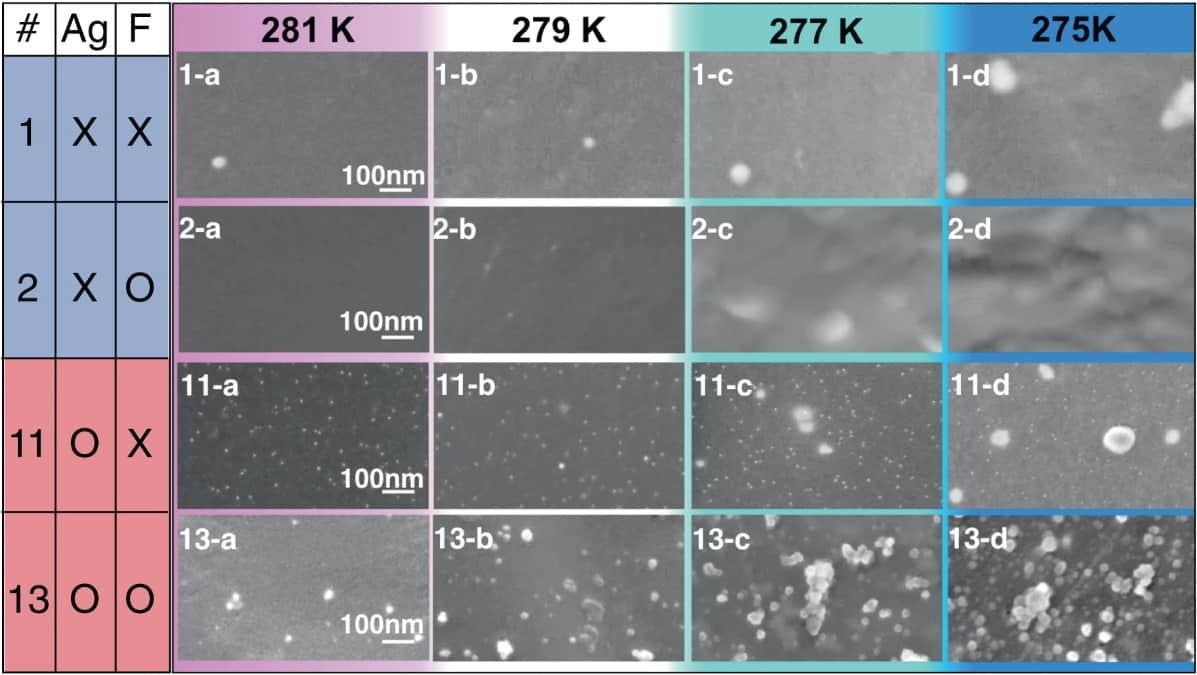
To study the effect, the team constructed experiments involving clathrate hydrates: water-based solids that resemble ice, in which polar or hydrophobic molecules are trapped within “cages” of hydrogen-bonded water molecules. After introducing different nanoparticle seeds to clathrate hydrates in liquid form, the researchers used a combination of scanning electron microscopy and X-ray absorption spectroscopy to directly image the formation of nucleation clusters.
When they added silver nanoparticles measuring between 5 and 10 nm across, the images revealed that clusters up to 30 nm in diameter would readily form around them. With some clusters even remaining at temperatures as high as 8°C, these nanoparticles clearly accelerated the crystallization process. In contrast, nanoparticles of unreactive noble metals – including palladium, gold and iridium – didn’t promote crystallization at all.
The discovery has shed new light on the nucleation mechanism, and the researchers anticipate that their results will provide a starting point for enhanced control over supercooling. By introducing the appropriate nanoparticles to clathrate hydrates, they envisage that it should be possible to develop more advanced latent heat storage materials. If achieved, these systems could be applied to technologies including solar energy and heat recovery systems for buildings.
The researchers report their findings in Communications Materials.



