Excitement is growing about the synergies between immunotherapy and radiation therapy, but there’s a long way to go before the clinical opportunities are realized at scale. Joe McEntee reports from ESTRO 2022 in Copenhagen
Better together? That’s the question that multidisciplinary oncology teams are seeking to answer across a burgeoning field of clinical study focused on combined-modality treatment regimes – not least, the convergence of advanced radiotherapy techniques with targeted immunotherapies that leverage the patient’s own immune system to eliminate cancer cells. The latter include antibodies capable of directly targeting tumour sites as well as so-called “immune checkpoint inhibitors” – drugs that help the immune system to “red-flag” and, in turn, attack cancer cells – which have transformed the treatment of several metastatic cancers, among them melanoma and non-small-cell lung cancer (NSCLC).
The case for convergence between the two disciplines lies in the tantalizing prospect – for which there is mounting evidence – that the immune system is able to extend the localized therapeutic impacts of radiotherapy with far-field (also called abscopal) antitumour responses elsewhere in the body. While observed abscopal effects are vanishingly rare when radiotherapy is used in isolation, the prospective win-win is evident nonetheless. In short: the potential for immunotherapy agents to scale the regional efficacy of radiation treatment, while on the flip-side localized radiation acts as an adjuvant for immunotherapy of solid tumours and lymphomas.
A winning combination
Those combined-modality synergies were front-and-centre last week at the ESTRO 2022 Annual Congress in Copenhagen, where several hundred delegates packed into Room D4 at the Bella Center for a joint ESTRO-ASTRO symposium entitled “Is integration with immunotherapy the new challenge for radiation oncologists?” Kicking off the debate (while joining the session remotely) was Silvia Formenti, a radiation oncologist at Weill Cornell Medicine in New York and one of the main-movers behind a paradigm shift in radiobiology, her efforts elucidating the role of ionizing radiation on the immune system while demonstrating the efficacy of combined radiotherapy–immunotherapy regimes in solid tumours.
Underpinning the emerging clinical opportunity is the use of radiation therapy to convert the tumour into an “in situ vaccine”, though the focus must always be on shifting the balance between radiotherapy-immunosuppressive versus pro-immunogenic signals. “The mindset I would encourage is not so much to use immunotherapy as a tool to enhance radiation response,” Formenti told delegates, “but rather to use radiation therapy as a tool to integrate with immunotherapy…The combination with immunotherapy is required to unleash the immunogenicity of radiotherapy.”
In terms of clinical implementation of the combined modalities, Formenti notes that radiation oncology teams have several parameters to think about as they strive for optimum treatment outcomes, including radiation field, radiation dose and fractionation; as well as factoring in the blood as an organ-at-risk (OAR). On this last point, there is plenty of evidence that radiation-induced lymphopenia (lowered white blood-cell count) is associated with poorer patient survival, with a consistent pattern across multiple tumour types including brain, oesophageal, NSCLC and pancreatic cancers.
As such, noted Formenti, it’s vital that pioneers of combined-modality approaches “adapt radiotherapy prescription and techniques to synergize with immunotherapy and sustain the patient’s fitness during treatment”. Inevitably, she concluded, that will mean radiotherapy implementations that emphasize a mix of hypofractionation, small field sizes and ideally fast dose rates.
Particle physics meets radiobiology
Those themes were echoed and developed by Alexander Helm, a research scientist in the biophysics division of GSI Helmholtzzentrum für Schwerionenforschung, a particle accelerator research facility in Darmstadt, Germany. Helm explored the synergies between particle therapy in combination with immunotherapy and began by flagging a much-cited US study from 2013 that modelled radiation dose to circulating lymphocytes in patients treated with radiotherapy for malignant gliomas. The US researchers determined that “a single radiation fraction delivered 0.5 Gy to 5% of circulating blood cells” such that “after 30 fractions 99% of blood cells had received ≥0.5 Gy” (Cancer Invest. 31 140).

Carbon ions team up with immunotherapy to tackle advanced tumours
Put simply, Helm explained, “radiotherapy compromises the immune system, so there is a need for reduced integral dose to healthy tissue, high dose rates and hypofractionation – and particles can be a match for that.” The physical advantages that come with particle therapy may extend to better sparing of lymphocytes and draining lymph nodes, such that circulating lymphocytes are available for mounting an efficient immune response. There are also hints of significant biological advantages triggered by particle therapy – for example, a broadening of the immunotherapeutic window of some cancers with low mutational loads.
“Preclinical evidence underpins the potential of particle therapy in combination therapies,” Helm concluded. “Furthermore, particle therapy can allow for efficient use of certain treatment modalities – for example, hypofractionation and stereotactic body radiotherapy – deemed to be beneficial.”
Betting on brachytherapy
A variation on the combined-modality approach – specifically, the opportunities presented by brachytherapy as an adjunct to immunotherapy – provided the narrative thread for Evert Jan Van Limbergen, a radiation oncologist and co-director at the Maastro radiotherapy clinic in the Netherlands.
“I think brachytherapy can provide some interesting approaches here, because the interstitial procedure – putting needles into the cancer as a part of the treatment procedure – means it’s a method of directly accessing human biopsies,” he explained. If the treatment involves three or four fractions, for example, the clinician can take those biopsies before each fraction and generate a timeline analysis to evaluate therapeutic impact of combination therapies (while mapping the localized dilution/concentration of injected drugs in the tumour using high-throughput analysis schemes).
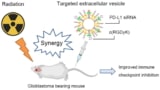
Immunotherapy plus a burst of radiation treats brain tumours in mice
At the same time, argued Van Limbergen, the unique dose rates associated with brachytherapy merit further scrutiny – from very low dose rate (vLDR) regimes (below 0.2 Gy/h) along the continuum through to high-dose-rate brachytherapy (above 12 Gy/h). Specifically, he highlighted an intriguing study in which a mouse model was treated with conventional radiotherapy or pulsed LDR brachytherapy (Cell Cycle 16 1171). The research showed that tissue excretion of TGF-β (an important immunosuppressant cytokine) is drastically lowered with the LDR brachytherapy scheme. “I think this is really an area that we might start looking at,” he added, “because maybe there will be a lot in there.”
The bottom line: while there have been some notable early successes from the integration of radiotherapy–immunotherapy combinations, there are still many unknowns to unpick before the clinical opportunities can be fully realized. “I would say we have a long way to go and it’s not [going to be] straightforward,” Van Limbergen concluded.



