
Researchers in Israel have engineered functional human spinal cord implants that they say could be used to help those with spinal injuries walk again. The technique transforms human tissue from elsewhere in the body using a process that mimics the embryonic development of the spinal cord. The implants enabled 80% of mice with chronic paralysis to walk again.
Every year, as many as half-a-million people around the world suffer a spinal cord injury. The vast majority of these are caused by traumatic events in which a sudden blow fractures, dislocates, crushes or compresses one or more vertebrae, or an object penetrates the body and cuts the spinal cord. Such traumatic spinal cord injuries have a catastrophic impact on health and quality-of-life.
In the weeks following the initial injury, progressive tissue damage usually occurs – caused by inflammatory and other responses – leading to the formation of scar tissue. This glial scar is a poor environment for cell growth and prevents tissue repair and natural neural regeneration. Strategies for treating paralysis focus on bridging this scar tissue to rewire the spinal cord. But it is not easy. Cells designed to regrow the spinal cord can be rejected by the patient’s immune system. They also lack a structural matrix in which to grow and struggle to thrive in the hostile micro-environment of the glial scar.
Tal Dvir, an expert in tissue engineering at Tel Aviv University, and his colleagues believe that the key could lie in growing spinal cord implants from the patient’s own tissue. A few years ago they developed a novel technique for making such implants. Using small samples of belly fat from patients they produced a thermo-responsive hydrogel and stem cells capable of generating several different cell types. They demonstrated that the hydrogel encourages the cells to grow in a natural way by producing several functional implants, including cardiac and spinal tissue.
Dvir explains that, like all tissues, the belly fat consists of cells and an extracellular matrix. “After separating the cells from the extracellular matrix we used genetic engineering to reprogramme the cells, reverting them to a state that resembles embryonic stem cells – namely cells capable of becoming any type of cell in the body,” he says. “From the extracellular matrix we produced a personalized hydrogel that would evoke no immune response or rejection after implantation. We then encapsulated the stem cells in the hydrogel and, in a process that mimics the embryonic development of the spinal cord, we turned the cells into 3D implants of neuronal networks containing motor neurons.”
In their latest work, published in Advanced Science, the researchers inserted laboratory grown spinal cord implants in mice. The implants were produced using extracellular matrix from pigs and stem cells generated from human belly fat. When mice received these implants immediately after spinal cord injury, 100% of them regained their ability to walk. Treating humans at this stage, however, would be impractical, as the extent of their injury would be unknown and it takes weeks to engineer the personalized implants.
To test a more realistic scenario, in which scar tissue has fully developed and recovery has plateaued, the researchers placed implants in mice six weeks after spinal cord injury. They explain that this is similar to a year post-injury in humans. Six weeks later, 80% of the mice could walk again.
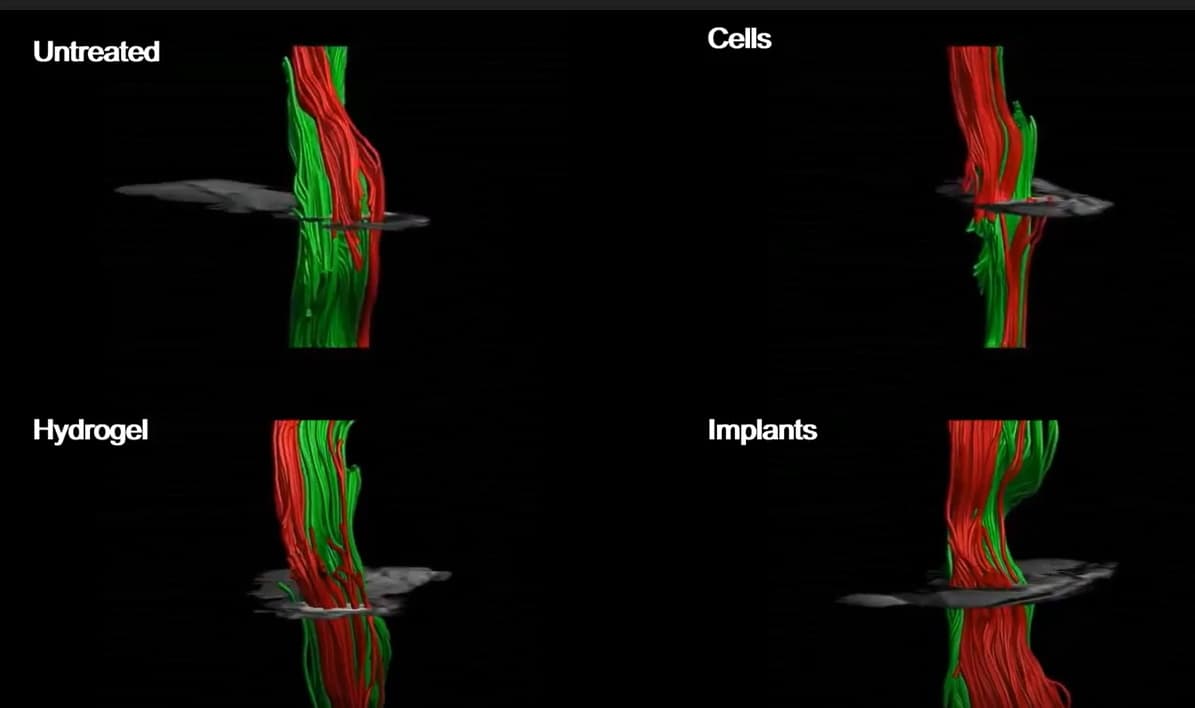
Recovery in the mice was rapid. Four weeks after treatment, imaging showed that intact nerve fibres were crossing the injury sites. In the weeks after receiving the implants, the mice demonstrated gradual improvements in co-ordination, feeling in their feet, and motor and sensory recovery, indicated by less missed steps as they walked. The animals exhibited significant recovery in various sensorimotor tests four weeks after they received the spinal cord implants.
Healing spinal cord injuries with graphene
“There are millions of people around the world who are paralysed due to spinal injury, and there is still no effective treatment for their condition,” Dvir says. “Individuals injured at a very young age are destined to sit in a wheelchair for the rest of their lives, bearing all the social, financial and health-related costs of paralysis. Our goal is to produce personalized spinal cord implants for every paralysed person, enabling regeneration of the damaged tissue with no risk of rejection.”



