Optogenetics – a technique that uses light to control the activity of neurons or other types of cell – has revolutionized our ability to manipulate and discern the mechanisms underlying brain function. Spinal cord activity underpins control of movement and several other basic physiological functions. But compared with the brain, optogenetics in the spinal cord presents a series of challenges that require the development of new light-delivery technologies.
For instance, implanting optical fibres is not a straightforward task, as the spine undergoes continuous displacement during natural movement. Therefore, one design challenge is the positioning of light-delivery sources over the very surface of the dura mater, the outer membrane that surrounds and protects the soft and dynamic spinal cord. Targeting deep intraspinal neurons presents additional obstacles. Light rapidly scatters when penetrating biological tissues. High irradiance, meanwhile, may result in local tissue heating due to light absorption, which can affect neuronal responses.
To overcome these challenges, and find a way to efficiently deliver light to the spinal depth of interest, researchers at the Swiss Federal Institute of Technology (EPFL) have developed a compliant, wireless-controlled optoelectronic implant customized for optogenetic studies of the spinal cord. They describe this novel implantable system in Nature Biotechnology.

Technology features
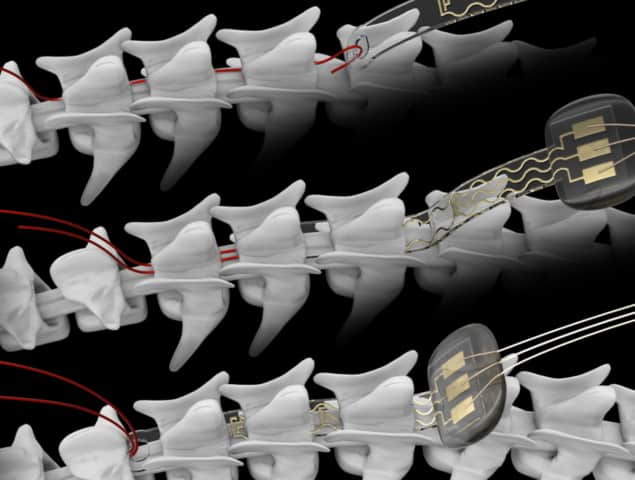
The new implant technology encapsulates miniaturized LEDs that can be switched on and off on the surface of a mouse’s spinal cord. A miniature head-mounted, wireless platform powers these micro-LEDs and performs customized on-chip processing to control light stimulation trains. This enables modulation of light pulses in real time, for instance, in response to the animal’s muscular activity or routine physiological signals. The wireless electronic circuit can control the duration and intensity of the emitted light with high precision.
In contrast to wire-based systems usually used for this type of research, wireless optoelectronic systems allow for unrestricted photostimulation of target neurons in the spinal cord of mice under untethered and ecological conditions.
In one key aspect of this work, the researchers, led by Grégoire Courtine and Stéphanie Lacour, used the micro-LED arrays to take advantage of the evolving library of experimental opsins (light-sensitive proteins activated by specific wavelengths) to target a broad range of cellular mechanisms.
They designed the micro-LED array to shift the emitted blue light toward a desired wavelength, enabling photostimulation using a broad spectrum of light, including red-shifted wavelengths that penetrate further into the spinal cord.
To optimize LED positioning and avoid tissue heating, the team ran simulations and performed in vivo recordings to quantify heating within the spinal cord for different levels of irradiance and cycles of photostimulation.
The researchers designed the new soft optoelectronic implant to be resilient and adaptable for long-term implantation and to meet the demanding mechanical dynamics of the spinal cord in freely behaving adult mice. Using a hybrid approach combining stiff LEDs and elastic interconnects, they created miniaturized implants that wrap around the surface and move along with the spinal cord.
Optogenetics implant is good news for incontinent rats
The team’s research has potential to pave the way for the development of therapeutic optogenetic applications. The ability to control the activity of spinal cord neurons with light could allow doctors to reduce pain and improve autonomic function.
Even though it may still be some time until these implants can be used clinically, Courtine finds optogenetics “revolutionary” and is eagerly anticipating further developments in biointegrated optoelectronic implants.