A recently discovered metal organic framework can act as a “quantum sieve”, with pores that selectively open for deuterium molecules at the right temperatures and pressures. The material was created in Germany by a team led by Stefan Kaskel and Thomas Heine at the Dresden University of Technology, and Michael Hirscher at the Max Planck Institute for Intelligent Systems in Stuttgart. Its development could reduce the considerable cost of isolating deuterium, which is an isotope of hydrogen that has several practical uses.
There are two stable isotopes of hydrogen and by far the most abundant is hydrogen-1 (H), which comprises a single proton. Hydrogen-2 (deuterium or D) comprises a proton and a neutron and accounts for just 0.015% of hydrogen nuclei in sea water. An isotope with two neutrons – tritium or T — is also found on Earth but is much rarer and unstable.
Pricier than gold
Deuterium has a range of uses from nuclear fusion to medical imaging and drug discovery. Today, chemical and physical processes are used to extract heavy water molecules – which contain two deuterium nuclei – from water. Then deuterium gas is created by electrolysis. The process is very expensive, with one gram of deuterium costing more than a gram of gold.
Some researchers believe that quantum sieves could do the job at much lower cost. A quantum sieve is a material that is porous on the molecular scale. The sieve is designed so that some types of molecules interact with the pores and pass through, while others do not.
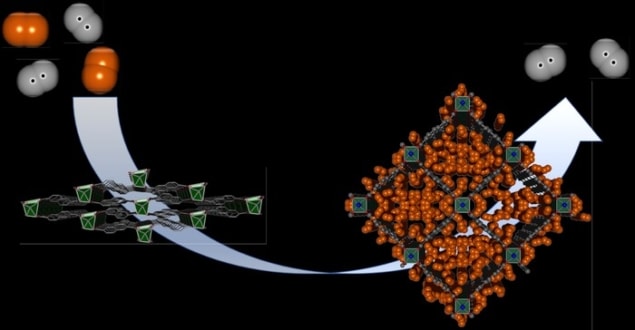
In 2012, researchers at Dresden created a new type of metal-organic framework (MOF). This is a material with metal ions that are linked together by organic ligands to form an orderly lattice.
Selective response
Called DUT-8, this material stood out for its flexibility, which enabled it to selectively respond to different external stimuli by adjusting its ligands – altering the size of its pores in turn. In the 2012 studies, researchers showed that DUT-8 prevented molecules of gaseous hydrogen from passing through – either at high pressures, or very low temperatures.
In their latest experiments, the researchers examined the material’s response to D2 – hydrogen molecules made from two deuterium nuclei. This involved probing the state of the pores using a combination of advanced imaging techniques including neutron diffraction, and low-temperature thermal desorption spectroscopy. The latter observes the extent to which molecules are removed from a surface as temperature increases.
First-principles calculations
At temperatures of about 23 K, and pressures of about 24 kPa, they observed that the DUT-8 pores opened in the presence of D2 – which allowed almost 12 times as many D2 molecules to pass through than H2. In parallel with their experiments, Hirscher’s team used first-principles calculations of the MOF’s properties, coupled with statistical thermodynamics, to simulate its isotope-selective properties.

Pulling water out of thin air
This allowed the researchers to describe the process by which the pores opened to D2 – while closing to H2 and HD. In addition, they predicted how the sieve would respond to tritium. Their simulations suggest that DUT-8 will open to molecules of T2 and DT, but remain closed for HT.
The team says that its combined results clearly demonstrate the promising potential for DUT-8 as an efficient way of separating the isotopes contained within gaseous hydrogen. Using this flexible quantum sieve, it could someday be possible to isolate large quantities of D2 gas at low cost.
The research is described in Science Advances.