Frost forms different patterns as it spreads across a surface depending upon the level of humidity, a novel imaging technique has revealed. These patterns range from complete surface coverage to fractal geometries.
Lukas Hauer, at the Max Planck Institute for Polymer Research in Germany, and his colleagues grew the frost on a microstructured surface covered in an array of micropillars spaced 30 µm apart. While this doesn’t replicate the types of surfaces that frost grows on naturally, it allowed the researchers to control the distribution of water droplets at the start of experiments. And condensation does tend to form in a pattern.
“When you have condensation on a surface, little droplets form on the surface and these droplets are usually separated by a certain distance and this distance is actually an average value. This is basically a property of the wettability of the surface,” explains Hauer. “So even if you have a smooth surface that is not microstructured, you will still see that droplets forming on the surface are separated by a characteristic distance.”
To see how frost changed with humidity, the researchers cooled the surface to −30°C and placed it in a sealed chamber. They then introduced nitrogen gas with a water vapour content of 14%, 24% or 34%. To observe the frost patterns, they used laser-induced fluorescence microscopy. The microstructure surface was infiltrated with silicon oil dyed with a fluorescent powder, which helped visualize the frost patches by increasing surface contrast.
This is the first time that this technique has been used for imaging frost and the researchers say that it allowed them to visualize frost formation in a more accurate and detailed way.
After the gas is introduced to the chamber, supercooled droplets condense on the micropillar tops, before randomly starting to freeze. These become frost nucleation sites, with frost patches growing out from them facilitated by water vapour. At the lowest humidity level (14%), most of the droplets evaporated quickly. This meant that few froze and those that did were unable to link with other droplets. Instead, they formed small spiky frost patches as droplets of water vapour froze to them.
At the highest humidity level of 34%, frost covered the surface. Evaporation was slower and almost all the droplets formed nucleated frost patches and ended up connecting to each other. Growing frost patches did halt as they approached each other, however, creating a small ditch that separated them.
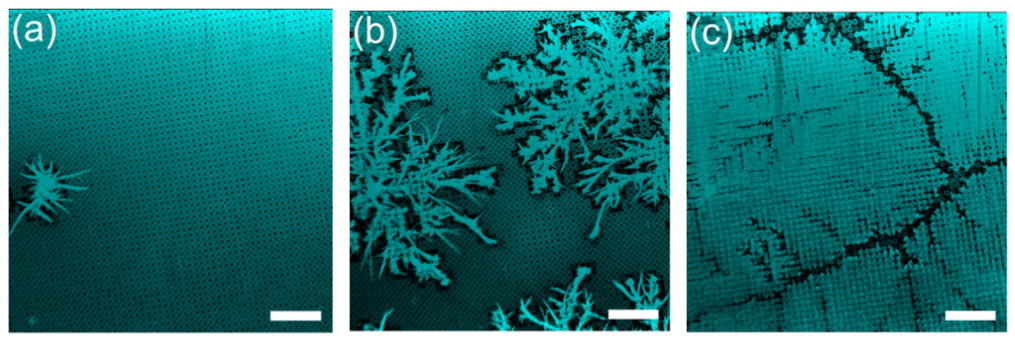
Intermediate humidity (24%) produced an intermediate level of evaporation. Frost patches spread out and connected with neighbouring liquid droplets, growing larger than in the low-humidity environment. But this did not always happen, as some droplets evaporated before the frost bridges could reach them. This led to a branching pattern with a fractal geometry. “It is a less dense frost; with the humidity, the density of the frost changes,” explains Doris Vollmer, also at the Max Planck Institute.
Hauer tells Physics World that the rate of evaporation is linked to the initial size of the water droplets. In less humid environments, small water droplets condensate on the micropillars and evaporate quickly.
The researchers also found that the number and size of frost patches can be tuned by surface temperature. At −25°C, with a relative humidity at 28%, a few large frost patches formed. When the temperature was reduced to −35°C, more but smaller frost patches formed, and at −45°C, the droplets all froze straight after condensation, creating almost as many frost patches as micropillars.

Ice crystals jump off surfaces in new electrostatic de-icing technique
Vollmer tells Physics World that improving our theoretical understanding of frost formation could help us reduce frosting and associated damage. She explains that understanding how humidity and temperature interact could allow us to develop different techniques for different parts of the world and different times of the year, which are linked to local environmental conditions, such as different anti-frosting surfaces.
“But it is difficult, because it is much, much more difficult to prevent frost formation than it to describe frost formation,” Vollmer says.
The researchers report their findings in Physical Review E.



