
Radiation therapy plays a vital role in the treatment of more than 50% of all cancer cases. But there’s a massive disparity in access to radiotherapy equipment as a function of a country’s income. In low- and middle-income countries, much of the population lacks access to radiation therapy. And in low-income countries, only 10% of patients have access to radiotherapy.
In a dedicated session at the AAPM Annual Meeting, speakers described some of the projects aiming to reduce the overall cost of delivering radiotherapy and improve access to effective cancer care for patients across the world.
Low-energy options
One potential way to lower the cost of radiotherapy could be to replace the expensive and bulky linear accelerators (linacs) used to deliver treatment with low-cost, lower-energy kilovoltage (kV) X-ray tubes, of the type used in diagnostic imaging systems.
Michael Weil from Sirius Medicine and Precision RT described just such a device: the “linear converging radiotherapy system”, or LCRS. “Why go to the effort of developing low-energy beams to treat cancer? The answer comes down to price, pictures and process,” he told the AAPM delegates.
While kV beams are historically considered unsuitable for radiotherapy, “low-energy beams can be manipulated in potentially useful ways for therapy,” said Weil. He described initial studies using high-Z contrast agents to enhance the dose to the target. Intra-lesion injection of such contrast under CT guidance, followed by delivery of kV X-rays at a modest skin dose, could provide “exquisite conformity of the high-dose region”.
Weil and collaborators used this approach to treat advanced cancers (under a palliation protocol), in 23 human patients and 80 veterinary patients with spontaneous tumours. They observed low dose to skin and significantly higher dose to deeper tumours. “Using conventional radiosurgery dosing regimens, we were confident that this approach could safely treat beneath the skin,” he said.
The team has now built a prototype LCRS, with the goals of cost reduction – using less expensive hardware and infrastructure – and dramatically improving the workflow. As the treatment source can also be used for real-time tracking, tighter margins can safely be used. The system also offers the possibility of performing planning and treatment in a single visit, with the ultimate aim of completing CT imaging, planning and treatment delivery in less than 1 hr.
The LCRS uses a magnetically swept electron beam aimed at a stationary tungsten target to create X-rays collimated in a single plane. The researchers tailored the beam geometry to spread out the entrance dose and concentrate the beam at the lesion. They also incorporated a flat-panel detector for CT, fluoroscopy and tomosynthesis. They note that the system’s footprint is similar to a standard CT system, though power and cooling requirements are greater.
To demonstrate the feasibility of treatment delivery with this kV source, the team simulated radiotherapy plans for breast and lung lesions (without contrast) using the kilovoltage arc technique (kVAT) and conventional volumetric modulated arc therapy (VMAT). For both cases, the most noticeable difference was a plateau in maximum dose for VMAT and a higher peak dose in the target with kVAT.
“We believe that this extra intra-lesion dose is beneficial in controlling hypoxic, relatively radioresistant central tumour cells,” said Weil.
Focused X-ray beams
Also looking to utilize a low-energy X-ray source, Mohammad Salehpour from the MD Anderson Cancer Center described a novel focused X-ray beam technology developed in collaboration with medical start-up company Convergent Radiotherapy and Radiosurgery (CRnR). The idea here is to combine a CT X-ray tube with an X-ray lens to create a converging 60 keV X-ray beam focused on the tumour target.
“An X-ray source such as used in a CT scanner will create a diverging beam of radiation,” Salehpour explained. “But if I had an X-ray lens, then I could create an intense beam at the focal point.”

CRnR has developed a proprietary focusing lens, composed of several concentric rings formed from tiles of single aluminium crystals, arranged to reflect the X-ray beam onto the target. Simulations of the 3D dose distribution showed a low-dose region slowly increasing to a high-dose focal spot in the centre before reducing again.
Salehpour notes that the depth–dose distribution resulting from a focused X-ray beam resembles the Bragg peak seen in proton therapy, with a higher intensity at depth than at the beam entrance or exit. For clinical purposes this enables delivery of high dose to the tumour and low dose to surrounding normal tissue. It’s also possible to stack these peaks to create a “photon spread-out peak”.
Salehpour and colleagues tested the X-ray lens in a water phantom containing dosimetry film, noting that the results reflected those seen in the simulations. Films irradiated by 60 keV X-rays through the lens exhibited dose circles at a depth of 20 mm, converging to a bright spot at 65–70 mm and then back to larger circles at greater depths.
“Considering the question ‘can we focus X-rays as we would focus rays of sunlight’ – the answer is yes,” said Salehpour. The team has now created a first-generation prototype with the X-ray tube and lens a housed in a robotic arm. They also added laser beams onto the robot, with the beams converging at the X-ray focal spot. As this system is based on diagnostic X-ray tubes, it requires far less shielding than other radiotherapy devices.
“Because it has a small footprint and low shielding requirements it can be made mobile and moved to different locations,” said Salehpour.
In addition to radiotherapy, where small shallow tumours are ideal treatment candidates, the technology could have many other applications. These include, for example, treating the eye condition age-related macular degeneration, combination with immunotherapy, cardiac ablation, or dose enhancement using gold nanoparticles.
Exploiting automation
Alongside the global inequality in access to radiotherapy equipment, there’s a similar gap in the availability of trained staff. To effectively expand access to radiotherapy, the introduction of lower cost radiotherapy systems must be accompanied by efforts to address of other resource issues. Tucker Netherton from the MD Anderson Cancer Center took a look at how artificial intelligence (AI) and automation could help narrow this radiotherapy gap.
Two approaches available immediately are “education” and “tele-radiotherapy”, he explained. In education, for example, the IAEA is currently developing a global curriculum for radiation oncologists, medical physicists and radiation therapists. Examples of tele-radiotherapy – defined as the use of telecoms and IT to provide radiotherapy support from a distance – include web-based treatment planning services and virtual tumour boards.
“By providing accessible treatment planning through tele-radiotherapy, automation can be used to offset task-related burdens for resource-limited clinics, saving time and increasing efficiency,” Netherton explained.
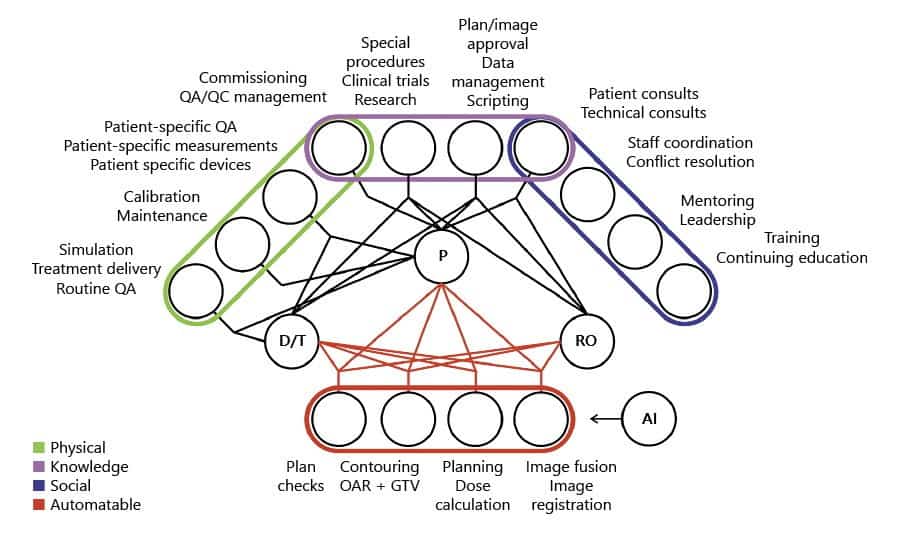
Examining which aspects of the treatment planning process could be automated, Netherton and colleagues concluded that AI can perform tasks such as plan checks, normal tissue and target volume contouring, planning and image registration. He noted that many vendors and researchers are already developing deep-learning based contouring tools with comparable performance to manual approaches.
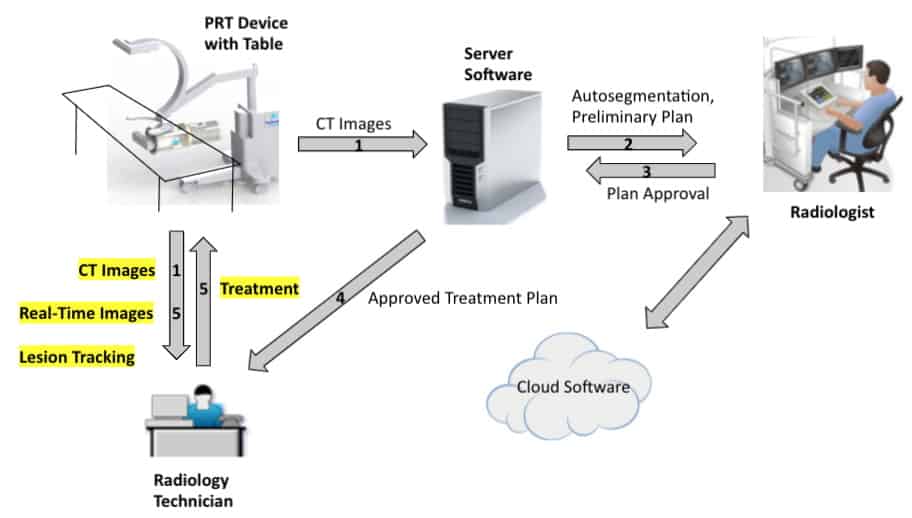
Automated treatment planning tools, meanwhile, could address the gap in resources by providing web-based access to planning services. With this goal, MD Anderson has created the Radiation Planning Assistant (RPA), a fully-automated contouring and radiotherapy treatment planning tool. The goal of the RPA, which is currently in research phase, is to make its services available to clinics at no cost. The clinic simply uploads the CT and plan order and the RPA returns a complete treatment plan. Impressively, the RPA can complete 483 cervix plans or 258 head-and-neck plans in 24 hr.
Affordable medical physics technologies tackle global health disparities
One major caveat to tele-radiotherapy is that Internet access is needed to access such tools. But Netherton notes that the number of Internet users is increasing globally and many efforts exist to “connect the disconnected”, including those by Google, SpaceX, Microsoft and the World Bank.
“Tele-radiotherapy can increase safety, efficiency and access to radiotherapy tools to provide resources to resource constrained clinics,” Netherton concluded.



