Mussels are famous for their ability to stick to a multitude of surfaces and now researchers in Canada and Germany have identified the molecular mechanisms used by mussels to produce robust adhesive threads. Using a range of imaging and spectroscopy techniques, a team led by Tobias Priemel at McGill University in Montreal found that the molluscs release fluid proteins into a network of microchannels in their feet, in coordination with separately stored metal ions.
To anchor themselves to their seashore habitats, mussels produce adhesive threads, called byssus. Once cured, the protein fibres of these structures become integrated with metal ions via mechanically stable cross-links – which are coordinated by an amino acid called DOPA.
By creating similar artificial structures, researchers aim to develop a new generation of bio-inspired polymers: with applications including self-healing materials; advanced coatings; and underwater adhesives. Currently, however the mechanisms employed by mussels to construct load-bearing cross-links within their byssus threads are largely unknown.
Microscopy and spectroscopy
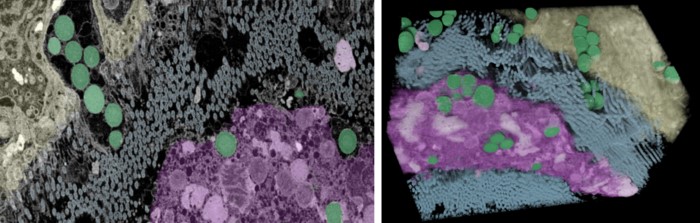
To learn more, Priemel’s team first investigated the byssus formation process using a combination of optical, electron, and X-ray fluorescence microscopy; then examined the chemical composition of the fibres using Raman spectroscopy.
They discovered that instead of drawing in metal ions from surrounding seawater, as had been previously thought, mussels concentrate and store ions of iron and vanadium within specialized storage particles. These particles are themselves contained within cells and held together by an as-yet unknown biomolecule. Within a separate stockpile of vesicles, mussels also carry concentrated, DOPA-rich proteins in a fluid form.
To produce their byssus threads, the team showed that mussels secrete proteins from these vesicles, into a complex network of tiny, interconnected microchannels their feet. In coordination with this process, metal storage particles are also delivered into the microchannel network, where they release their metal ions – possibly through a pH-driven process.
Exploiting mussel technology to glue tissue
As they diffuse and spread through the dense protein fluid, the ions are then coordinated by DOPA molecules to form cross-links. In the process, the mixture coalesces to form mechanically stable threads, featuring strong protein-metal bonds.
For the first time, this mechanism allowed Priemel’s team to explain how mussels can strongly adhere to almost any solid surface, even in seawater conditions. With a deeper knowledge of this process, researchers could soon find it far easier for researchers to replicate byssus filaments that are just as strong as those found in nature. Through future research, the researchers also hope to shed new light on why mussels use vanadium ions in particular – which are exceptionally rare in nature.
The study is described in Science.



