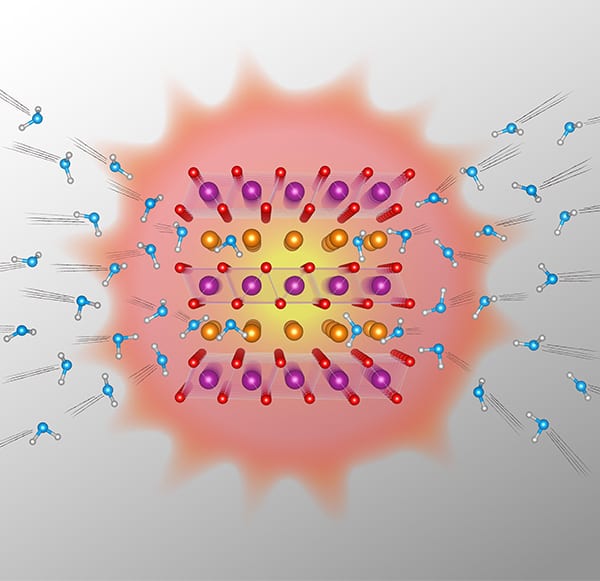
A form of manganese oxide that incorporates water between its layers could make an ideal heat-storage material. The newly-synthesized oxide is similar to the common mineral birnessite in its composition, and the Japan-based researchers who created it say that its volumetric energy density exceeds 1000 MJ/m3 – close to the theoretical maximum and the highest among all minerals that “lock” other molecules within their structure.
Led by Tetsu Ichitsubo and Norihiko Okamoto of Tohoku University’s Institute for Materials Research and Rigaku Corporation, the researchers fabricated their layered manganese oxide with crystalline water, forming a so-called intercalated compound designated as δ-type K0.33MnO2 ⋅ nH2O. The water molecules between the layers are easily lost through heating, and the team used X-ray diffractometry and transmission electron microscopy to study how the material’s structure changed as it heated or cooled.
Fast and reversible transformation
The team found that the water disappeared when the material was heated to 200°C. The resulting dehydrated material then released this absorbed heat energy when cooled to 120°C and exposed to moisture in the ambient surroundings. According to Ichitsubo, this mechanism is very advantageous for heat storage. It is also very fast, reversible and the material’s structure remains stable.
“The water is almost frozen in the material to start with and changes to vapour form once it is released,” he explains. “We can exploit the large entropy gap between these solid and gas phases for heat storage.”
A host of heat-storage applications
The researchers analysed the material’s heat storage properties using humidity-controlled thermogravimetry and differential thermal analysis together with differential scanning calorimetry. According to Ichitsubo, the calorimetric energy density they measured is high enough to be practical for heat-storage applications where space is limited.

Surmounting supercooling to improve heat storage technologies
One possibility, he suggests, would be a rooftop system in which the energy collected from solar heat in the daytime gets released at night by exposing the dehydrated manganese oxide compound to moisture in its surroundings. Another option could involve automobiles. “Here, the waste heat can be stored while driving the vehicle and then released on demand for warming up the engine or the battery at a later time,” he tells Physics World.
The researchers, who detail their work in Nature Communications, say that their layered manganese oxide is easy to synthesize by thermally decomposing potassium permanganate (KMnO4). They now plan to increase the amount of water they can accommodate in the material. “We will do this by optimizing the concentration of the interspersed ion species in our compound and hence further increase its volumetric energy density,” Okamoto reveals.
- This article was amended on 19 May 2022 to remove a reference to “crystals of water”, which is not a synonym for “crystalline water”; the former refers to water in its crystalline form (in other words, ice), whereas the latter refers to water molecules incorporated into the crystalline lattice of another material.