
An in-depth knowledge of human organs and how they form is pivotal to understanding how diseases affect these tissues. Unfortunately, it remains inherently difficult to study living organs as they develop inside us. Thus, over the last decade, scientists have developed organoids: three-dimensional organ models that self-organize to mimic true organ behaviour. Such organoids show potential to revolutionize how we study human disease and how organs themselves grow at their genesis.
The functional architectures of organs develop as cells self-organize in response to external stimuli in their microenvironment. Inspired by this concept, a research group led by Matthias Lutolf of EPFL, Switzerland controlled the self-organization process of intestinal stem cells to show how substrate microtopography could be used to control the size and shape of the resulting bioengineered organoids. As detailed in Science, the researchers utilized this understanding to build organoids resembling the geometry of intestinal epithelium and to determine the roles of tissue geometry in cell fates.
As the processes behind organoid formation are largely stochastic, current methods for engineering these complex tissues lack reproducibility. For example, it would be difficult to replicate native intestinal tissues that feature varied cell types across and according to their complex architecture.
To overcome this obstacle, Lutolf’s group utilized soft lithography to microstructure a 3D hydrogel with cavities of defined size and shape, then filled the cavities with purified intestinal stem cells. The researchers followed the differentiation of and protein expression in these cells to connect cell-type localization to the surrounding tissue geometry. They demonstrated that tissue shape leads to a “prepatterning” of epithelial cells with varying protein activity, which, in turn, drives the formation of specific cell types according to the region’s topography.
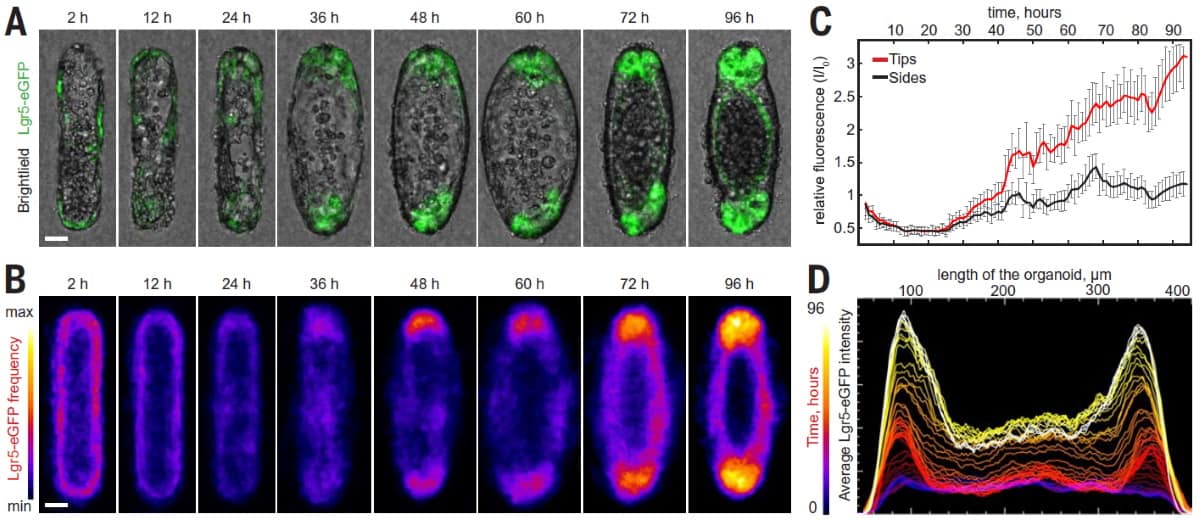
Having demonstrated the deterministic mechanisms by which tissue geometry governs cell fate, the researchers aimed to exploit the effect to mimic native tissue. They fabricated hydrogel substrates with an architecture that resembled native intestinal mucosa, then seeded the structures with intestinal stem cells. Within 48 hours of seeding, these cells formed a monolayer over the surface. Subsequently, the cells differentiated according to the local geometry and as predicted by the discovered mechanisms.

Finally, the team used these printed organoids to explore shedding, a physiological process that cells use to deal with proliferative crowding. Shedding becomes dysregulated under pathological conditions such as inflammatory disease. The researchers found that shedding was associated with the appearance of an actin ring at the cell interface. They also replicated the excessive shedding induced by an inflammatory factor, showing the efficacy of the approach. The in vitro system allowed tracking of shed cells for downstream analysis, which is not feasible with other approaches.
By connecting the geometry of a cell’s microenvironment to its gene expression, Lutolf’s team realized how substrate patterning might make stem cell differentiation deterministic rather than stochastic. Moving forward, this creative strategy presents a means to examine the development of complex organoids and to use the tissues for translational research. As philosopher Eric Hoffer said: “creativity is the ability to introduce order into the randomness of nature”.



