Thyroid nodules – small lumps that form within the thyroid gland – are relatively common, particularly among women. Most are harmless, but a small percentage of such nodules are cancerous. Currently, preliminary screening of thyroid nodules is performed by physical examination aided by ultrasound imaging and a biopsy. However, existing ultrasound procedures for assessing thyroid nodules suffer from low sensitivity and specificity. This lack of effectiveness could impact the screening results, leading to inaccurate diagnosis, missed cancers or false positives that may result in unnecessary surgeries.
LUCA – a beam of hope
To address this problem, a team of multidisciplinary scientists has created LUCA: a laser and ultrasound co-analyser for thyroid nodules. This innovative technology, developed by scientists in the LUCA consortium and coordinated by the Institute of Photonic Sciences (ICFO) in Barcelona, aims to provide enhanced information during thyroid screening, resulting in better diagnosis and improved patient care. The group is developing a simple, low-cost, multimodal device that combines the use of near-infrared light and medical ultrasound to improve the screening of thyroid nodules for cancer.

The LUCA device, described in Biomedical Optics Express, combines two photonic technologies – near-infrared time-resolved spectroscopy (TRS) and diffusion correlation spectroscopy (DCS) – with multifunctional ultrasound imaging, eliminating the need for biopsy. What distinguishes the LUCA device from conventional ultrasound systems is that in addition to the ultrasound examination, which provides anatomical information, LUCA also provides physiological information.
Firstly, the device measures the optical properties of the underlying thyroid tissue using the TRS module, which utilizes short laser pulses (roughly 100 ps) of varying wavelength. In tests on a healthy volunteer, the team was able to acquire quantitative information regarding physiological and cellular structures in the thyroid. Secondly, the DCS module, which uses a continuous-wave laser source to illuminate the tissues, measures the intensity of blood flow. By combining these two modules, the researchers can obtain complementary data on tissue haemodynamics, oxygen metabolism and structure – information that could reduce the uncertainty in the diagnosis of thyroid nodules.
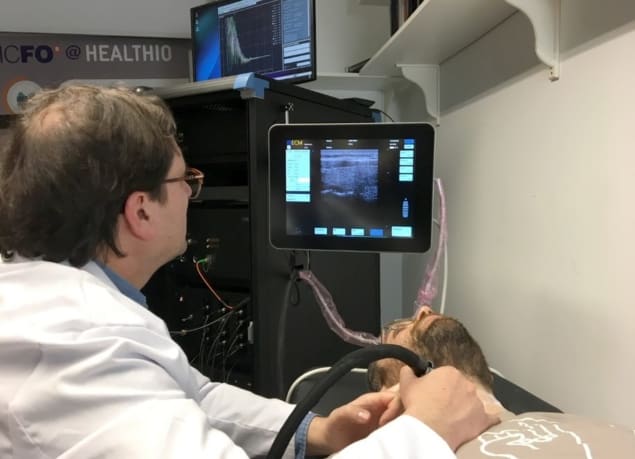
LUCA’s precision test
To ensure the alignment of the different technologies within the LUCA prototype, the team created a unique multimodal probe. The probe includes both a standard ultrasound transducer and optical fibres, to allow simultaneous acquisition of the optical and ultrasound signals. The system also includes an interactive display system incorporating several functionalities.
To verify the precision of LUCA in the reproducibility of data, the team monitored the thyroid lobe of a healthy volunteer, performing five independent measurements of the same lobe on four days over the course of two weeks. The average scanning time was approximately one minute for each measurement. The researchers observed slight variations in DCS and TRS values between repeated measurements. However, they believe that the variations are inconsequential, given the high quality of ultrasound images acquired by the device.
Specifically, the device could determine haemodynamic properties with a precision of better than 3% in a single measurement and an image reproducibility outstripping 10% during in vivo measurements over several days.
Towards clinical acceptance
Senior author Turgut Durduran, head of the medical optics group at ICFO, explains that for LUCA to be accepted for clinical use, it must first be standardized through calibration and quality assurance procedures. In establishing the clinical usability of the LUCA device, the team also validated the TRS and DCS through independent phantom tests. They used a 32-cm head-and-neck phantom that mimics the human anatomical structure to test the linearity, accuracy and reproducibility of the LUCA device. Tests of the TRS module using this phantom validated its suitability for in vivo studies.
Meanwhile, the team tested the DCS module, which measures haemodynamic parameters, by using it to measure the Brownian diffusion coefficient of a liquid phantom. The capacity of the LUCA device to accurately distinguish the concentration properties in the phantom further demonstrated its clinical usability.
Durduran says that the team is now using the device in a clinical environment and has tested it on 18 healthy volunteers and diagnosed thyroid nodules in 47 patients. The study revealed the potential of the LUCA device for identifying nodules as benign or malignant. The researchers note that these nodules were originally depicted as indistinct cases using conventional ultrasound screening.
Currently, the team is striving to improve the precision and accuracy of the optical data analysis to enhance disease detection and prognosis. The researchers believe that the LUCA device provides an innovative tool with the potential to reduce costs related to thyroid cancer screening, efficiently diagnose thyroid cancer nodules, and improve the thyroid cancer screening process in comparison with conventional ultrasound.



